Capsaicin Nanoparticles as Therapeutic Agents against Gliomas
Evelin Martínez-Benavidez 1, Inocencio Higuera-Ciapara 2, Sara Elisa Herrera-Rodríguez 3, Ofelia Yadira Lugo-Melchor 1 *, Francisco Martín Goycoolea 4, Francisco Javier Guerrero Jazo5
1 Unidad de Servicios Analíticos y Metrológicos, Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco, A. C. (CIATEJ). Guadalajara 44270, Jalisco, México.
2 Universidad Anáhuac Mayab, S.C. Mérida 97302, Yucatán, México.
3 Unidad Sureste, CIATEJ. Mérida 97302, Yucatán, México.
4 School of Food Science and Nutrition. University of Leeds. Woodhouse Ln, Leeds LS2 9JT. UK.
5 Departamento de Neurocirugía, Hospital Civil “Dr. Juan I. Menchaca”. Guadalajara, 44340, Jalisco, México.
* Corresponding author. E-mail: ylugo@ciatej.mx Tel.: +52 3333455200
Received: Nov. 3, 2020; Accepted: Dec. 2, 2021; Published: Dec. 16, 2021
Citation: Evelin Martínez-Benavidez, Inocencio Higuera-Ciapara, Sara Elisa Herrera-Rodríguez, Ofelia Yadira Lugo-Melchor, Francisco Martín Goycoolea, and Francisco Javier Guerrero Jazo, Capsaicin Nanoparticles as Therapeutic Agents against Gliomas. Nano Biomed. Eng., 2021, 13(4): 433-445.
DOI: 10.5101/nbe.v13i4.p433-445.
Abstract
Capsaicin is an alkaloid molecule with outstanding biological activity. Several reports have shown that capsaicin exerts significant antitumoral effects in several cancer cell lines, including gliomas. However, its application has been very limited due to its hydrophobicity, low affinity, and short life span. Gliomas are a heterogeneous group of brain malignant tumors with increasing prevalence worldwide. Standard therapy against these tumors generally includes resection by surgery, radiation, and chemotherapy or their combination. However, elicitation of tumor resistance to chemical or radiation treatments remains one of the main challenges to be resolved, particularly in the case of glioblastomas. Nanotechnology is an innovative approach to the treatment of Central Nervous System diseases and especially to gliomas treatment. Indeed, the use of nanotherapeutic formulations offers several advantages over the conventional methods of drug delivery therapy. In this review, we analyzed the current literature regarding the development of capsaicin-loaded nanoparticles as a promising approach for the treatment of malignant brain tumors.
Keywords: Nanoencapsulation, Capsaicin, Glioblastoma, Drug delivery
Introduction
Natural products continue to be a key resource in the treatment of diseases. According to Newman and Cragg [1], 49% of the small molecules approved for cancer treatment from the 1940s to 2014 are natural products or molecules derived therefrom. Among them, capsaicin (CAP) (8 methyl-N-vanillin-trans-6-nonemaide) stands out as a hydrophobic molecule with great therapeutic potential and low cost. The CAP molecule comprises three distinct regions, each with a specific biological relevance, i.e., the aromatic hydrophilic region (vanillin), the dipolar amide region, and the hydrophobic region (octanylic chain) [2, 3]. It possesses a highly reactive methoxy phenol group [4]. According to Yang et al., [5] CAP forms specific chemical interactions with the Ca2+ channel through hydrophobic attractions, suggesting dynamic conformational transitions that may be responsible for bioactivity. Numerous additional reports have documented the underlying molecular action mechanisms [6, 7] and its potential in the treatment of a variety of cancers [8]. This review addresses the use of CAP nanoparticles for the treatment of gliomas.
Capsaicin and the Central Nervous System (CNS).
CAP is a specific agonist of the Transitory Receptor Potential Vanilloid (TRPV-1), a tetrameric membrane protein with four identical subunits and a member of the family of Transient Receptor Potential (TRP) Receptors [9]. TRPV1 is expressed in the sensory neurons, as well as in numerous non-neuronal tissues including the blood vessels. Nagy et al., [10] showed that TRPV-1 is expressed extensively in the brain and the epidermis, among other tissues. CAP is well known for its ability to act through the intracellular union to the TRPV1 receptors in order to deploy excitatory, desensitizing, and neurotoxicity effects [11]. The description of the structure-activity relationship of CAP and several of its analogs suggest that the vanillin region and the amide linkage are essential for the pharmacological activity on TRPV1 and thus, to display its excitatory capacity, while it is presumed that the aliphatic chain is essential for maximum potency. CAP binds with a strong affinity to the intracellular S2-S4 of TRPV1 protein, mediated by interactions between the vanilloid group and the benzene ring [6]. In order to understand the configuration of the linkage between CAP and its interaction with TRPV1, Yang et al., [5] developed an innovative hybrid approach combining the computational coupling and the functional studies. According to their work, the TRPV1 and CAP structures obtained from cryoTEM imaging clearly show conformational reorganizations near the union site, with CAP linked in a “tail-up, head-down” configuration, Binding is mediated by both hydrogen bonds and van der Waals interactions. Upon binding, CAP stabilizes the open state of TRPV1 by “pull-and- contact” with the S4-S5 linker (Fig. 1). In contrast, the vanilloid and amide groups form specific interactions that bind the ligand to the receptor [12]. TRPV1 is one of the main receptors involved in pain sensation, thus it is considered a therapeutic target against pain and inflammation and has been widely used [13-15]. According to Abdel-Salam et al., [15] CAP stimulates the vagal afferent fibers involved in the signaling of the immune system to the brain. The application of painful stimuli on the skin by CAP results in the activation of the medial thalamic pathway to the frontal lobe, including the area of the prefrontal cortex [16]. TRPV1 has also been identified in the hippocampus, striatum, hypothalamus, and cerebellum in the brain of humans and rats [17]. According to Kauer and Gibson [18] the activation of TRPV1 in the CNS improves synaptic transmission and plasticity as well as memory formation in the hippocampus. In experimental diabetes models, Araya et al., [19] showed that peripheral and central TRPV1 play a key role in heat hyperalgesia, while Xu et al., [20] studied the beneficial effect of CAP on preventing Alzheimer's disease via tau changes in the hippocampus of type 2 diabetes rats, which could be related to TRPV1 activation. Activation of TRPV1 by CAP has also been reported to stimulate the spontaneous release of glutamate in the locus coeruleus [21]; moreover, microinjection of CAP into the substantia nigra has been used to induce degeneration of mesencephalic dopaminergic neurons [22]. Farrell et al., [23] identified regional brain activations evoked by CAP inhalation. These regions include the insula, mid cingulate cortex, prefrontal, parietal, and premotor regions and the cerebellum. In the brainstem, CAP produced activations in respiratory-related regions of the dorsal pons and lateral medulla (Fig. 2). TRPV1 can become reactive under stress conditions and glial cell lesions, thus showing an increase in hypertrophy, cytokine production, and secretion, as well as changes in gene expression. In addition to the neurons, TRPV1 is also found in astrocytes and microglia and the emergent studies have implied TRPV1 in various aspects of glial function [25]. Also, when associated with increases in intracellular calcium, the overactivation of TRPV1 may become toxic to cells. Ho et al., [26] have shown high levels of intracellular calcium, as well as subsequent mitochondrial damage and apoptosis induced by CAP. Several reports have shown that CAP exerts significant antitumoral effects in several cancer cell lines, i.e., pancreas [27], lung [28], prostate [29], breast [30], colorectal [31], stomach [32], bladder [33], osteosarcoma [34], nasopharyngeal carcinoma [35], cholangiocarcinoma [36], melanoma [37], fibrosarcoma [38] and in human glioblastoma [11, 39, 40]. The molecular basis for such effects has not been completely elucidated, however, some are related to activation CAP receptors or regulating other signaling pathways. The anticancer mechanisms are mainly related to anti‐proliferation, induction of apoptosis and autophagy, anti‐angiogenesis, and anti‐metastasis [41]. A major aspect of CAP action is its high specificity to cancer cells while allowing healthy cells to thrive unaffected [42].
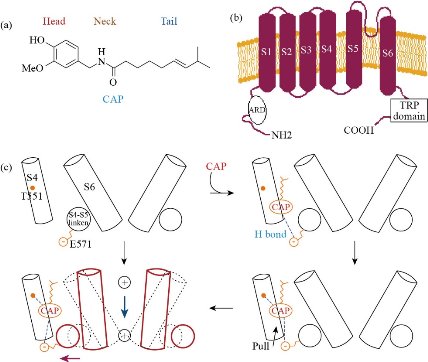
Fig. 1 (a) Chemical structure of capsaicin. (b) Schematic diagram of a TRPV1 subunit; figures taken from [5], with some modifications c) Schematic diagram summarizing capsaicin binding and activation of the TRV1 channel; figure reprinted with permission from [5, 12].
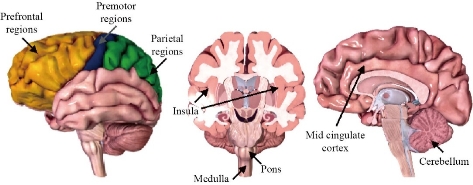
Fig. 2 Regions of the brain activated by capsaicin inhalation [23]. Images: BIODIGITAL [24]
CAP activity against glioblastoma
Gliomas have been classified according to their malignancy to affected cells in astrocytoma (astrocytes), oligodendrogliomas (oligodendrocytes), and ependymomas (ependymal cells). Accordingly, they are classified into various intensity categories into Low Grade (grade II) or High-Grade (Grades III and IV) Malignant Gliomas. The most aggressive form of glioma is Glioblastoma (Grade IV) which is characterized by uncontrolled tumor cell proliferation, causing necrotic areas and diffuse infiltration [43-46]. In 2016, the Word Health Organization (WHO) incorporated molecular diagnostic criteria in the classification for infiltrating gliomas, including mutation of isocitrate dehydrogenase, deletion of 1p/19q chromosome, and histone mutations [47]. Glioblastoma, also known as Grade IV astrocytoma, occurs more frequently in males and it is one of the most aggressive human cancers with an average expectancy of survival of around one year after diagnosis [45, 48]. Intracellular signaling pathways for tumor growth and proliferation are key to an invasion of healthy tissue and for stimulation of blood vessel proliferation. Much effort in the drug development process has been focused on interference with such mechanisms [49]. Many factors that influence the process of tumorigenesis, proliferation, and invasion have been identified, for example, many growth factor pathways and cytokines are involved in the phenotype of malignant gliomas. Knowledge about the role of TRP channels in brain tumor growth and progression has been evolving rapidly [50]. Angiogenesis has been widely recognized as a key event in glioma progression [51]. Indeed, neovascularization in brain tumors is highly correlated with its biological aggressiveness, malignancy degree, and clinical recurrence and outcome. It is also inversely correlated with post-operatory survival. Recently, a direct connection has been shown between the expression changes in the TRP channels during tumoral angiogenesis [52]. Given the importance of angiogenesis in glioma growth and progression, TRP channels have become an increasingly promising target for therapeutic agents capable of inducing anti-angiogenesis. In this regard, the “vascular segmentation strategy” [53] will be a very promising therapy. Nersesyan et al., [54] assessed the TRPV1 expression in different glioma cell lines and determined the correlation between the expression of the protein with age and sex in tissue samples obtained from patients with glioblastoma. Brito et al., [73] described the role of TRPV1 in the normal physiology and physiopathology of various body organs. These authors highlight that, drugs such as CAP, which targets this channel, could be of clinical importance. It is through the activation of TRPV1 that CAP seems to exert many of its beneficial effects, since the TRPV1 receptors are found in numerous types of tissues [73, 74]. Tumoral angiogenesis is a physiological response to hypoxia in response to an increase in tumor mass and also the result of critical mutations which activate a transcriptional program leading to angiogenesis [75]. Calcium (Ca2+) is an important secondary messenger and its entry through the plasma membrane affects angiogenesis [53]. Several reports indicate that angiogenic growth factors such as the Vascular Endothelial Growth Factor (VEGF) and the Fibroblastic Growth Factor (FGF) can activate the TRP channels at the transcriptional and post-transcriptional levels, causing an increase in the endothelial Ca2+, which in turn modulates the transduction signaling pathways which regulate angiogenesis [76]. According to Kale et al., [77] the ion channels are implicated in oncogenesis. In this context, numerous in vivo experiments targeted to various ion channels in cancer models illustrate the great potential of this approach [78, 79]. TRPV1 is expressed in a wide variety of glial cells, especially in microglia and astrocytes [25]. Glial cells carry out immunologic activities in response to biochemical challenges through the secretion of proinflammatory cytokines and chemokines [80]. The inflammation modulates the expression and activity of TRPV1 in the CNS [81]. Amantini et al., [11] suggest that the molecular characterization of the TRPV1 channel in glioma tumors could be a useful indicator for their evolution and prognosis as well as a key target for new therapeutic approaches. Thus, CAP offers great potential from a theoretical and experimental viewpoint. Indeed, several studies have reported the effects of CAP on human glioblastoma (Table 2). Gil and Kang [40] assessed the effect of CAP on inhibition and cell differentiation in human glioma cells. Their results indicated that CAP induces apoptosis through the induction of mitochondria-mediated caspase cascades via down-regulation of Bcl-2 and up-regulation of Bax. It was also observed that CAP increases the levels of caspase-3 and -9. It is well known that caspase-3 activation plays a role in the induction of apoptosis and is frequently preceded by the expression of Bcl-2 and Bax via the mitochondrial pathways. It was also shown that CAP induces terminal differentiation through the expression of genes related to such differentiation. On the other hand, Jeon et al., [82] showed that CAP induces inhibition of human glioma cell viability. Apoptosis mechanisms were related to the mitochondrial (Bcl-2/Bax) and activation of the MAPK pathways (Kinase Protein Activated by Mitogens). This protein is involved in processes leading to the death of various cancer cell lines. A different study showed that CAP can induce apoptosis and autophagy in glioblastoma cells. In this case, the authors verified the role of apoptosis and autophagy in U251 cells after capsaicin treatment by using the autophagy inhibitor 3-methyladenine. The results showed that inhibition autophagy could increase the expression of P53 (a suppressor of tumor growth) and Puma- in U251 cells, thus concluding that CAP could induce apoptosis by autophagy inhibition [83]. A further study by Kim et al., [46] in different cell lines of malignant glioma showed that CAP is a potent sensitizer of apoptosis induced by the TRAIL ligand (Tumor Necrosis Factor Related Apoptosis Inducing Ligand), a potent apoptotic stimulator; tumor cells are significantly more sensitive to such apoptosis than normal cells [84]. On the other hand, Xie et al., [85] showed that the CAP and di-hydro CAP apoptotic effects in U251 cells are associated with the generation of Reactive Oxygen Species (ROS), increased concentrations of calcium (Ca2+), mitochondrial depolarization, the release of cytochrome C in the cytosol resulting in the activation of Caspase-3 and -9, and these effects were further confirmed by observations of the anti-tumor effects of CAP and di-hydro CAP in vivo in a U251 cell murine tumor xenograft model. Qiao et al., [39] studied the role of peroxynitrite on C-6 glioma cells apoptosis; they found that CAP stimulated an increase in superoxide and nitrite causing an increase in peroxynitrite inside the glioma cells concomitant to the increase in the levels of nitrotyrosine proteins. Table 2 summarizes the in vitro mechanisms and doses of CAP reported in the literature, while Fig. 3 shows the main molecular target of CAP in glioma cells. In addition to the activity in glioblastoma cells, it has been shown that CAP has a notable effect on the integration of cells tight junctions and permeability [87, 88]. Previous in vivo studies demonstrated that the use of CAP may result in the reversible opening of the Blood-Brain Barrier (BBB), which is a key limiting factor in the bioavailability of therapeutic agents to the brain [89]. The BBB is a physical and enzymatic barrier responsible for preserving brain homeostasis. It selectively inhibits the permeabilization of high molecular weight compounds with a negative charge and low lipid solubility [90]. Only a few chemical moieties have been found to trespass the BBB [91]. In vitro studies using murine models have shown that CAP can open the tight junctions of the endothelial monolayer in a dose-dependent reversible manner [89, 92, 93]. This opens the possibility that CAP is used not only as a therapeutic agent but also as a candidate for the development of drug vehicles. However, its use remains limited due to its hydrophobicity, low affinity, and short life span [94]. Standard therapy against gliomas includes surgery, radiation, and chemotherapy or their combination. However, elicitation of tumor resistance to chemical or radiation treatments remains one of the main challenges to be resolved, particularly in the case of gliomas [43, 95]. A further complication stems from the collateral harm on healthy cells given the low specificity of the therapeutic agents. Thus, a major area of research interest is the achievement of more effective drug systems with less collateral damage [96]. A major tool to achieve this goal is the use of nanotechnology. Indeed, new formulations based on nanoparticles allow more specific drug delivery on cell ligands so that accumulation of the therapeutic agent reaches minimum inhibitory concentrations at a faster pace and with high specificity toward cancer cells [97]. In addition, it is possible to design nanosystems to carry specific chemical conformations able to recognize specific cell receptors resulting in a higher drug load accumulation inside the tumor with diminished secondary effects [96, 97].
Table 1 Reported Glioma receptors
|
Receptor |
Types of receptor |
Study model |
Ref |
|
Protease-activated receptor 2 |
(PAR) 2 |
U87 cells and human glioma tissue |
[55] |
|
Adenosine receptor (AR) |
A1, A2A, A2B, A3 |
Glioma cells (C6, U373MG, U87MG, U138MG, ADF) |
[56] |
|
Metabotropic P2Y nucleotide receptors |
P2Y1 – P2Y12 |
C6 cells |
[57] |
|
Ionotropic receptors P2X |
P2X7 |
C6 cells |
[57] |
|
Receptor tyrosine kinase |
RTK |
Human glioma tissue |
[58] |
|
Epidermal growth factor receptor |
EGFR |
Human glioma tissue |
[59] |
|
Platelet-derived growth factor |
PDGFR |
Human glioma tissue |
[59] |
|
Vascular endothelial growth factor |
VEGFR-1, VEGFR-2 |
Human brain tumors, glioma cells (C6, U87MG) |
[60] |
|
Discoidin domain receptor 1 |
DDR1 |
Glioma cells (U87MG, G140), human glioma tissue |
[59] |
|
Neurotrophic tyrosine kinase receptor type 1 |
TrkA |
Glioma cells (U251, C6-2B), human glioma tissue |
[59] |
|
Cannabinoid receptor |
CB1, CB2 |
Glioma cells (U87MG, U373MG, C6), tissue samples |
[61] |
|
Transforming growth factor-β |
TGF-β |
Glioma cells (U373MG, A172), tissue samples |
[62] |
|
Hepatocyte growth factor/scatter factor |
HSF/SF |
Glioma cells (U251MG, U373MG, GL261) |
[63] |
|
Fibroblast growth factor receptor |
FGFR |
Glioma cells (HGG, SF188, KNS42), patient-derived cell lines (IN1591, IN2017, IN2356, IN2688, IN1520) |
[64] |
|
Insulin-like growth factor receptor |
IGFI, IGFII, IGF-IR, IGR-IIR |
Human glioma tissue, normal brain |
[65] |
|
Cytokines |
|||
|
Interleukin-13 receptor |
IL-13R |
Human glioma tissue, normal brain |
[66] |
|
Folate receptor |
FR- |
Human glioma tissue |
[67] |
|
Other receptors |
|||
|
Transferrin receptor |
TfR2 |
Glioma cells (TB10, U87MG, T98G, U251) |
[68] |
|
Integrins |
v3, v5 |
Glioma cells (U87MG, SF763) |
[69] |
|
Tenascin |
TNC |
Human glioma tumor |
[70] |
|
Adhesion receptors |
|||
|
|
CD44 |
Human glioma tissue |
[71] |
|
CD90 |
Human glioma tumor, glioma cells (U87MG, U251) |
[72] |
|
Table 2 In vitro studies evaluating the action mechanisms of CAP in gliomas
|
Cell lines |
Effective dose (mM) |
Anti-cancer mechanisms |
Ref |
|
A-172 |
200-250 |
Induced apoptosis by the reduction in the basal generation of ROS |
[86] |
|
C6 |
50 - 200 |
Induced apoptosis by formation of peroxynitrite. |
[39] |
|
U373, U87, FC1, FLS |
50 |
Induced apoptosis mediated by TRPV1 and requires p38 MAPK activation. |
[11] |
|
A-172 |
50 - 100 |
Induced apoptosis through down-regulation of Bcl-2 and activation of caspase-3 and terminal differentiation. |
[40] |
|
U251MG, U87MG, SNU-444, U251MG |
200 |
Induced apoptosis mediated TRAIL via DR5 upregulation and surviving down-regulation. |
[46] |
|
U87MG |
200 |
Induced apoptosis mediated via p-38 MAPK and mitochondrial (Bcl-2/Bax) pathway. |
[82] |
|
U251 |
100 |
Induced apoptosis, autophagy, and activation of the expression of P53. |
[83] |
|
U251 |
200 |
Induced apoptosis via ROS and Ca2+ mediated mitochondrial pathway. |
[85] |
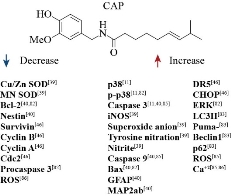
Fig. 3 Molecular target of CAP in glioma cells.
Nanoparticles against Glioblastoma
As noted above, the use of nanotherapeutic formulations offers significant advantages over the conventional methods of drug delivery, i.e., faster accumulation of the drug in the cancer tissue thus increasing its therapeutic effect in a shorter time, decrease in systemic toxicity, and the possibility of surface functionalization to achieve a higher degree of specificity according to the molecular binding site. Also, nanoparticles can circulate throughout the body without risk of stroke and penetrate most cell compartments without difficulties [98, 99]. Numerous investigations have shown that it is possible to control the distribution profiles of anticancer drugs by trapping in submicronic colloidal systems, thus increasing the antitumoral effects while reducing secondary systemic damages [100]. Additionally, nanoparticles provide the possibility of delivering the drug to the brain as they increase the drug concentration inside or at the luminal surface of the BBB cells, compared to what can be achieved after the systemic administration of a free drug. This favors passive diffusion of the drug and subsequent uptake by target cells. There are several mechanisms by which nanoparticles can be transported to the brain, endocytosis is one of the main ones [101, 102]. Nanotechnology is an innovative approach to the treatment of CNS diseases and, particularly, for the treatment of gliomas. Indeed, several research reports have been published on nanoparticles for brain drug delivery [102]. Among the strategies, lipid-based carriers, polymer-based carriers, and inorganic carriers are the most widely studied. The physicochemical properties of such systems, as well as their in vitro stability and, in some cases, the assessment of their biological properties, have been described. Table 3 presents examples of biopolymer-based nanoparticle systems developed for the treatment of malignant glioma brain tumors. An excellent review on the nanoparticle drug delivery systems to target high-grade gliomas has been recently provided by Frosina [118]. In this review, two chitosan-based nanosystems are described. The first one was reported by Fang, et al., [111] who used chitosan to protect temozolomide (TMZ) from degradation and increase its penetration to gliomas by using the tumor-targeting peptide chlorotoxin which binds to glioma initiating cells. These nanoparticles had an average diameter of less than 100 nm and exhibited sustained stability in cell culture media for two weeks. According to the research carried out by Saboktakin et al., [106] methotrexate loaded glycol-chitosan and dextran sulfate nanoparticles represent a good alternative for brain tumor treatment. Yang et al., [108] formed electrostatic complexes preparing an aqueous mixture with chitosan and hyaluronic acid. Such complex was found to present a higher uptake in C6 glioma cells. Hernández-Pedro et al., [119] detailed the development of a nanovector made with a supra-paramagnetic iron oxide core covered with polyethylene glycol (PEG) grafted with chitosan and polyethanolamine for the treatment of gliomas. Specificity for the tumor was achieved with siRNA and chlorotoxin peptide. Kievit et al., [120] developed a nanofiber system based on chitosan-polycaprolactone to study the migration of glioblastoma and the topographical change induced by its growing cells. This nanofiber substrate offers a new tool to study the effect of anti-migratory agents on malignant cells. Irani et al., [121] developed an innovative tertiary system comprising TMZ chitosan loaded and gold nanoparticles which were incorporated onto polyurethane nanofibers. This system proved effective in inhibiting the growth of U87 glioblastoma cells in vitro. Also, Muzzarelli and Muzarelli [122] prepared methotrexate-loaded O-carboxymethyl chitosan nanoparticles and assessed the release of the anticancer agent in various media. These authors have developed a wide variety of nanosystems using covalently bound bioactive onto chitosan matrixes. On the other hand, improved release through convection has been explored by Saltzman [123] for delivery of chemotherapeutic agents for the treatment of gliomas in animal models, thus achieving significantly longer survival periods. The best results were obtained when target cells where glioma stem cells. Castro [124] proposed the development of high-density lipoprotein synthetic nanoparticles to administer chemotherapeutic agents to gliomas in animal models. Such nanoparticles are transported through the cell surface receptor SR-B1 and can induce glioblastoma tumor regression. Kuo and Hsu, [114] reported on the use of colloidal solid-lipid cationic nanoparticles with melanotransferrin and apolipoprotein E (ApoE) on their surface to transport the anti-mitotic drug doxorubicin through the BBB for glioblastoma treatment. Nanoparticles were applied to endothelial microvascular cells, astrocytes, and U87MG cells with good results. The best encapsulation results for sustained release were obtained with 10% stearyl-amine for sustained release and optimal drug delivery. Chitosan-blended nanoparticles offer many advantages for the delivery of CAP to gliomas as they can overcome the BBB by adsorptive transcytosis, a key transport mechanism for positively charged molecules [125]. Glioblastoma can also be inhibited by blocking the CK2 protein (Kinase 2 Protein) and the overexpression of the EGFR and the EGFRvIII (Epidermal Growth Factors). When such therapy approach is followed by the stimulation of anti-tumor immunity by antibodies against Antigen 4 (CTLA-4), associated with cytotoxic lymphocytes or the Lymphocyte Surface Receptor (CTL), the efficacy of the nanoconjugates treatment will be increased [126]. Additionally, a multicomponent, flexible chain nanoparticle system composed of three iron oxide nanospheres and a liposome loaded with a chemotherapeutic agent was developed by Karathanasis [127]. This system provides a linear chain assembly to facilitate the release of the agent on the walls of the glioma blood vessels. The application of a low external radiofrequency field thereafter allows the rapid release of the chemotherapeutic agent due to the rapid mechanical disruption of the liposome and its beneficial on-site action.
Table 3 Biopolymer-based nanoparticle systems against glioblastoma
|
Biopolymer |
Drug |
Effects |
Ref |
|
PLGA |
Transferrin |
The nanoparticles were found to be highly absorbed and endocytosed by the cells using an energy-dependent process. |
[103] |
|
Angiopep-PEG-PLC |
Paclitaxel |
The nanoparticles enhanced accumulation in the Glioma bed and infiltrating margin of intracranial U87MG glioma tumor-bearing in vivo model were observed by real-time fluorescence image. |
[104] |
|
N-isopropyl acrylamide vinylpyrrolidone and acrylic acid |
Curcumin NanoCurcTM |
The nanoparticles can inhibit malignant brain tumor growth through the modulation of cell proliferation, survival, and stem cell phenotype. |
[105] |
|
Glycol chitosan, Dextran sulfate |
Methotrexate |
The physicochemical properties of resulting particles were investigated, evidencing the contribution of these nanoparticles for brain targeting. |
[106] |
|
Glutathione-PEG |
Doxorubicin |
In vitro BBB Transwell™ study showed significantly higher permeation of the doxorubicin-loaded NPs compared with the free doxorubicin solution through the coculture of rat brain endothelial (RBE4) and C6 astrocytoma cells. |
[107] |
|
Hyaluronic acid/chitosan |
Curcuminoid |
The electrostatic complex showed stronger dose-dependent cytotoxicity against C6 glioma cells and higher uptake in C6 glioma cells. |
[108] |
|
mPEG-PLC |
Capsaicin |
The nanoparticles can cross the blood-brain barrier and showed a remarkable inhibition on the growth of U251 cells |
[94] |
|
PLGA |
Loperamide |
The nanoparticles can efficiently cross the BBB, with high crossing efficiencies when their surface is functionalized with an active targeting moiety. |
[109] |
|
PLGA |
Paclitaxel and curcumin |
The nanoparticles can efficiently penetrate the BBB and enhance brain delivery efficiency, as demonstrated in both in vitro and in vivo studies. The system exhibited improved glioma therapy efficacy yet with reduced adverse toxicities. |
[110] |
|
Chitosan |
Temozolomidechlorotoxin and biotin |
The nanoparticles exhibited sustained stability in cell culture media for two weeks and can cross the BBB and deliver temozolomide into avascular region of the brain. |
[111] |
|
MPEG-PCL |
Honokiol and doxorubicin |
The nanoparticles could efficiently suppress glioma cell proliferation and induce cell apoptosis in vitro. |
[112] |
|
Cerium Oxide |
|
The nanoparticles had a cytotoxic effect on anaplastic astrocytoma (grade III glioma) cells while the same concentration did not show cytotoxicity on microvascular endothelial cells used as stromal cell models. |
[113] |
|
Anti-melanotransferrin and apolipoprotein E |
Doxorubicin |
The nanoparticles improved the permeability of Dox across the BBB and enhanced the efficiency in inhibiting the multiplication of U87MG cells. |
[114] |
|
PLGA |
Doxorubicin |
The results demonstrated that doxorubicin-loaded PLGA nanoparticles are efficiently internalized into the human glioma cells via clathrin-mediated endocytosis, then accumulate in lysosomes and release the doxorubicin |
[115] |
|
Lactoferrin-hyaluronic acid/chitosan |
Curcuminoid |
The results showed that nanoparticles were preferentially taken up by brain capillary endothelial cells. After crossing the BBB, the nanoparticles remained largely intact and were more effective in targeting glioma cells (C6). |
[116] |
|
Transferrin-DSPE-PEG2K |
Temozolomide and bromodomain |
Tumor-bearing mice treated with nanoparticles loaded with temozolomide and the bromodomain inhibitor JQ1 have decreased tumor burden and prolonged survival. |
[117] |
Capsaicin Nanoencapsulation
From the above discussion, it is clear that CAP can be tumor prevention and anticancer molecule with significant effects. However, its application has been very limited due to its hydrophobicity which causes low solubility in physiological fluids and a short half-life [94]. The following section describes some reports in which several CAP-loading nanosystems have been developed and evaluated to find suitable carriers to prolong the drug retention of CAP in the blood circulation and allow active targeting of specific cancer cells for enhanced, precise delivery and target specificity [41]. Mantellero et al., [128] used in vitro studies with Franz diffusion cells to study the CAP release kinetics using different solid formulations. Results showed a higher CAP release from the nanoencapsulated formula when compared to other formulations, while Amruthraj et al., [129] prepared CAP-capped silver nanoparticles that were found to be compatible with blood cells in hemagglutination tests. Furthermore, Xing et al., [130] reported the formulation of gelatin-acacia nanocapsules loaded with CAP through complex coacervation with hydrolysable tannins. Such nanocapsules had a mean diameter of 300-600 nm and were able to allow a 20.81% CAP load, with 81.17% efficiency. Sharma et al., [131] used solid-lipid nanoparticles to encapsulate CAP which exhibited a mean size of 100 nm, and prolonged release of the bioactive compound was observed for a period of 14 h. Bejrapha et al., [132] prepared capsicum oleoresin loaded nanocapsules in a gelatin matrix to determine the effect of the freezing process in the absence or presence of excipients, on the stability of such nanocapsules during freeze-thawing and freeze-drying procedures. Zhu et al., [133] prepared capsaicin-loaded liposomes (52.2 ± 1.3 nm mean diameter) for oral administration. The in vivo pharmacokinetic study and irritation tests showed an enhanced bioavailability and reduced inflammation in a murine gastric mucosa model. This same research group formulated a polyvinylpyrrolidone (PVP) /sodium cholate /phospholipid micellar system for oral administration which significantly improved the CAP oral bioavailability and showed a reduced irritation on the gastric mucosa [134, 135]. Peng et al., [136] prepared CAP-loaded methoxy poly(ethyleneglycol)-poly(ε-caprolactone) nanoparticles aimed at improving solubility and bioavailability and reducing the side effects of CAP, while Cirino et al., [137] evaluated the effectiveness of liposomes to deliver CAP to protect rodent bladder tissue against CAP-induced inflammation. Studies in our laboratories [138] have addressed the effect of the degree of N-acetylation of chitosan on the biophysical properties, colloidal stability, and encapsulation efficiency of CP in chitosan-based nanocapsules. This system comprised an oily core, lecithin, and a polymer layer and it proved to be a promising platform for the effective administration of peptides, lipophilic drugs, and vaccines. Additional research on this same system by Kaiser et al., [87] compared the cytotoxicity of free CAP versus the chitosan-CAP nanocapsules on epithelial cell lines (MDCK-C7) and their effect on the integrity of the tight junctions and cell permeability. This study demonstrated that when CAP is encapsulated, it can be applied at a concentration of 500 μM without compromising the viability of MDCK-C7 cells as compared to the free molecule. Giri et al., [139] evaluated the effectiveness of CAP-loaded nanoliposomes in protecting the liver from oxidative stress. The phospholipid vesicle (nanoliposomes) had a mean diameter of 277.7 nm. In this study, they observed that free CAP produced mild protection, while liposomal CAP exerted a significant effect on reducing liver oxidative stress. Jiang et al., [94] evaluated the ability of the CAP-loaded methoxy polyethylene glycol-poly(caprolactone) (mPeg-PLC) nanoparticles to cross the BBB and the uptake of these nanoparticles in the glioma cells and its ability to inhibit cell proliferation in human glioblastoma. The efficacy of the CAP-loaded nanoparticles against tumor cells was significantly higher than the CAP by itself, especially at low concentrations (p<0.05). After the absorption of CAP-loaded nanoparticles in tumor cells by endocytosis at low concentrations, CAP was released slowly into the cytosol resulting in increased efficiency. Recently, we evaluated the antiproliferative activity of CAP-loaded nanoemulsions and chitosan nanocapsules in two glioblastoma cell lines (H4, U118MG). The CAP-loaded nanosystems exhibited a significant reduction in cell viability compared to their unloaded counterparts [140]. Although more research is required to understand better the mechanisms of action and the mechanisms for drug delivery at the active site, these nanosystems hold promise in preclinical and clinical applications [102].
Conclusions
The advantages of nanobiotechnological approaches in the development of suitable carriers to extend CAP retention in the blood, thus facilitating active targeting to specific cancer cells can provide new therapeutic solutions for the treatment of CNS diseases and to the treatment of gliomas. Nanoparticles will continue to play a significant role in drug delivery, and numerous research reports have been published on specific nanosystems for brain drug delivery. In turn, different CAP-loaded nanosystems are successful in improving bioavailability, increasing half-life, and reducing irritation at research level. Although more research is required to better understand the underlying mechanisms of action and the mechanisms for drug delivery at the active site, these nanosystems hold promise in preclinical and clinical applications.
Acknowledgements
This work was supported by CONACYT (Project FON.INST./44/2016 and A1-S51264 of Ciencia Básica).
Conflict of Interests
The authors declare that no competing interest exists.
References
[1] D.J. Newman, G.M. Cragg, Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 2016, 79: 629-61.
[2] A. Alberti, V. Galasso, B. Kovac, et al., Probing the molecular and electronic structure of capsaicin: a spectroscopic and quantum mechanical study. Journal of Physical Chemical A, 2008, 112: 5700-11.
[3] C.S. Walpole, R. Wrigglesworth, S. Bevan, et al., Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 3. The hydrophobic side-chain "C-region". Journal of Medicinal Chemestry, 1993, 36: 2381-9.
[4] A.C. Bort, M.C. Morell, A.D. Ramos, et al., Efecto de la capsaicina en el metabolismo de células de hepatocarcinoma. Dianas, 2014, 3: e20140909.
[5] F. Yang, J. Zheng, Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein & Cell, 2017, 8: 169-77.
[6] I. Díaz-Laviada, N. Rodríguez-Henche, The potential antitumor effects of capsaicin. Progress in Drug Research, 2014, 68: 181-208.
[7] A.A. Oyagbemi, A.B. Saba, O.I. Azeez, Capsaicin: a novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian Journal of Cancer, 2010, 47: 53-8.
[8] K.C. Pramanik, S.K. Srivastava, Role of capsaicin in cancer prevention: Role of capsaicin in oxidative stress and cancer. Springer, Dordrecht, Netherlands, 2013.
[9] M.J. Caterina, M.A. Schumacher, M. Tominaga, et al., The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature, 1997, 389: 816-24.
[10] I. Nagy, P. Sántha, G. Jancsó, et al., The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. European Journal of Pharmacology, 2004, 500: 351-69.
[11] C. Amantini, M. Mosca, M. Nabissi, et al., Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. Journal of Neurochemistry, 2007, 102: 977-90.
[12] F. Yang, X. Xiao, W. Cheng, et al., Structural mechanism underlying capsaicin binding and activation of the TRPV1 ion channel. Nature Chemical Biology, 2015, 11: 518-24.
[13] M. Huang, G. Cheng, H. Tan, et al., Capsaicin protects cortical neurons against ischemia/reperfusion injury via down-regulating NMDA receptors. Experimental Neurology, 2017, 295: 66-76.
[14] K.N. Browning, T. Babic, G.M. Holmes, et al., A critical re-evaluation of the specificity of action of perivagal capsaicin. Journal of Physiology, 2013, 591: 1563-80.
[15] O.M. Abdel-Salam, R.F. Abdel-Rahman, A.A. Sleem, et al., Modulation of lipopolysaccharide-induced oxidative stress by capsaicin. Inflammopharmacology, 2012, 20: 207-17.
[16] D.H. Lee, K.J. Lee, K.I. Cho, et al., Brain alterations and neurocognitive dysfunction in patients with complex regional pain syndrome. Journal of Pain, 2015, 16: 580-6.
[17] E. Mezey, Z.E. Tóth, D.N. Cortright, et al., Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97: 3655-60.
[18] J.A. Kauer, H.E. Gibson, Hot flash: TRPV channels in the brain. Trends in Neurosciences, 2009, 32: 215-24.
[19] E. I. Araya, C.F.M. Nones, L.E.N. Ferreira, et al., Role of peripheral and central TRPV1 receptors in facial heat hyperalgesia in streptozotocin-induced diabetic rats. Brain Research, 2017, 1670: 146-55.
[20] W. Xu, J. Liu, D. Ma, et al., Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS One, 2017, 12: e0172477.
[21] S. Marinelli, C.W. Vaughan, M.J. Christie, et al., Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. Journal of Physiology, 2002, 543: 531-40.
[22] S.R. Kim, D.Y. Lee, E.S. Chung, et al., Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. Journal of Neuroscience, 2005, 25: 662-71.
[23] M.J. Farrell, L.J. Cole, D. Chiapoco, et al., Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. NeuroImage, 2012, 61: 1324-35.
[24] BioDigital: 3D Human Visualization Platform for Anatomy and Disease, 2019, https://human.biodigital.com/index.html.
[25] M.C. Marrone, A. Morabito, M. Giustizieri, et al., TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nature Communications, 2017, 8: 15292.
[26] K.W. Ho, N.J. Ward, D.J Calkins, TRPV1: a stress response protein in the central nervous system. American Journal of Neurodegenerative Disease, 2012, 1: 1-14.
[27] K.C. Pramanik, S.K. Srivastava, Apoptosis signal-regulating kinase 1-thioredoxin complex dissociation by capsaicin causes pancreatic tumor growth suppression by inducing apoptosis. Antioxidants & Redox Signaling, 2012, 17: 1417-32.
[28] K.C. Brown, T.R. Witte, W.E. Hardman, et al., Capsaicin displays anti-proliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS One, 2010, 5: e10243.
[29] A. Mori, S. Lehmann, J. O'Kelly, et al., Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Research, 2006, 66: 3222-9.
[30] N.H. Thoennissen, J. O'Kelly, D. Lu, et al., Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene, 2010, 29: 285-96.
[31] J. Jin, G. Lin, H. Huang, et al., Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. International Journal of Biological Sciences, 2014, 10: 285-95.
[32] A. Sarkar, S. Bhattacharjee, D.P. Mandal, Induction of apoptosis by eugenol and capsaicin in human gastric cancer AGS cells--elucidating the role of p53. Asian Pacific Journal of Cancer Prevention, 2015, 16: 6753-9.
[33] M.H. Lin, Y.H. Lee, H.L. Cheng, et al., Capsaicin inhibits multiple bladder cancer cell phenotypes by inhibiting tumor-associated NADH oxidase (tNOX) and sirtuin1 (SIRT1). Molecules, 2016, 21: 849.
[34] T. Jin, H. Wu, Y. Wang, et al., (2016) Capsaicin induces immunogenic cell death in human osteosarcoma cells. Experimental and Therapeutic Medicine, 2016, 12: 765-70.
[35] Y.T. Lin, H.C. Wang, Y.C. Hsu, et al., Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. International Journal of Molecular Sciences, 2017, 18: 1343.
[36] A. Wutka, V. Palagani, S. Barat, et al., Capsaicin treatment attenuates cholangiocarcinoma carcinogenesis. PLoS One, 2014, 9: e95605.
[37] D.H. Shin, O.H. Kim, H.S. Jun, et al., Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Experimental & Molecular Medicine, 2008, 40: 486-94.
[38] Y.P. Hwang, H.J. Yun, J.H. Choi, et al., Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Molecular Nutrition & Food Research, 2011, 55: 594-605.
[39] S. Qiao, W. Li, R. Tsubouchi, et al., Involvement of peroxynitrite in capsaicin-induced apoptosis of C6 glioma cells. Neuroscience Research, 2005, 51: 175-83.
[40] Y.G. Gil, M.K. Kang, Capsaicin induces apoptosis and terminal differentiation in human glioma A172 cells. Life Sciences, 2008, 82: 997-1003.
[41] S. Zhang, D. Wang, J. Huang, et al., Application of capsaicin as a potential new therapeutic drug in human cancers. Journal of Clinical Pharmacy and Therapeutics, 2020, 45: 16-28.
[42] J.K. Lau, K.C. Brown, A.M. Dom, et al., Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis, 2014, 19: 1190-201.
[43] T. Glaser, I. Han, L. Wu, et al., Targeted nanotechnology in glioblastoma multiforme. Frontiers in Pharmacology, 2017, 8: 166.
[44] K. Suk, Proteomic analysis of glioma chemoresistance. Current Neuropharmacology, 2012, 10: 72-9.
[45] L. Emdad, Z.A. Qadeer, L.B. Bederson, et al., Is there a common upstream link for autophagic and apoptotic cell death in human high-grade gliomas? Neuro-Oncology, 2011, 13: 725-35.
[46] J.Y. Kim, E.H. Kim, S.U. Kim, et al., Capsaicin sensitizes malignant glioma cells to TRAIL-mediated apoptosis via DR5 upregulation and survivin downregulation. Carcinogenesis, 2010, 31: 367-75.
[47] R. Chen, M. Smith-Cohn, A.L. Cohen, et al., Glioma subclassifications and their clinical significance. Neurotherapeutics, 2017, 14: 284-97.
[48] S. Lepannetier, N. Zanou, X. Yerna, et al., Sphingosine-1-phosphate-activated TRPC1 channel controls chemotaxis of glioblastoma cells. Cell Calcium, 2016, 60: 373-83.
[49] ABTA. 2012. Glioblastoma y astrocitoma maligno. American Brain Tumor Association. Available from: https://www.abta.org/wp-content/uploads/2018/03/glioblastoma-y-astrocitoma-maligno.pdf
[50] M. Alptekin, S. Eroglu, E. Tutar, et al., Gene expressions of TRP channels in glioblastoma multiforme and relation with survival. Tumor Biology, 2015, 36: 9209-13.
[51] W. Liang, B. Guo, J. Ye, et al., Vasorin stimulates malignant progression and angiogenesis in glioma. Cancer Science, 2019, 110: 2558-72.
[52] T. Smani, L.J. Gómez, S. Regodon, et al., TRP Channels in angiogenesis and other endothelial functions. Frontiers in Physiology, 2018, 9: 1731.
[53] G. Santoni, M.B. Morelli, M. Santoni, et al., New deals on the transcriptional and post-transcriptional regulation of TRP channel target genes during the angiogenesis of glioma. Journal of Experimental and Integrative Medicine, 2011, 1: 221-234221.
[54] Y. Nersesyan, S. Asuthkar, K.K. Velpula, et al., Role of TRPV1 channels in glioma cell viability and survival. Biophysical Journal, 2015, 108: 124a.
[55] R. Luo, X. Wang, Y. Dong, et al., Activation of protease-activated receptor 2 reduces glioblastoma cell apoptosis. Journal of Biomedical Science, 2014, 21: 25.
[56] S. Ceruti, M.P. Abbracchio, Adenosine signaling in glioma cells. Advancesin Experimental Medicine and Biology, 2020, 1202: 13-33.
[57] D. Wypych, J. Barańska, Cross-talk in nucleotide signaling in glioma C6 cells. Advancesin Experimental Medicine and Biology, 2020, 1202: 35-65.
[58] S.E. Little, S. Popov, A. Jury, et al., Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumor heterogeneity. Cancer Research, 2012, 72: 1614-20.
[59] M. Nakada, D. Kita, L. Teng, et al., Receptor tyrosine kinases: principles and functions in glioma invasion. Advancesin Experimental Medicine and Biology, 2020, 1202: 151-178.
[60] D.A. Reardon, S. Turner, K.B. Peters, et al., A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. Journal of the National Comprehensive Cancer Network, 2011, 9: 414-27.
[61] A. Ellert-Miklaszewska, I.A. Ciechomska, B. Kaminska, Cannabinoid signaling in glioma cells. Advancesin Experimental Medicine and Biology, 2020, 1202: 223-41.
[62] S. Peñuelas, J. Anido, R.M. Prieto-Sánchez, et al., TGF-β Increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer cell, 2009, 15: 315-27.
[63] B. Badie, J. Schartner, J. Klaver, et al., In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth factor/scatter factor. Neurosurgery, 1999, 44: 1077-83.
[64] N. Egbivwie, J.V. Cockle, M. Humphries, et al., FGFR1 expression and role in migration in low and high grade pediatric gliomas. Frontiers in Oncology, 2019, 9: 103.
[65] C. Maris, N. D'Haene, A.L. Trépant, et al., IGF-IR: a new prognostic biomarker for human glioblastoma. British Journal of Cancer, 2015, 113: 729-37.
[66] W. Debinski, D.M. Gibo, B. Slagle, et al., Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. International Journal of Oncology, 1999, 15: 481-6.
[67] H. Wu, Y. Zhan, Y. Qu, et al., Changes of folate receptor -α protein expression in human gliomas and its clinical relevance. Zhonghua Wai Ke Za Zhi, 2014, 52: 202-7.
[68] A. Calzolari, L.M. Larocca, S. Deaglio, et al., Transferrin receptor 2 is frequently and highly expressed in glioblastomas. Translational Oncology, 2010, 3: 123-34.
[69] N. Skuli, S. Monferran, C. Delmas, et al., Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Research, 2009, 69: 3308-16.
[70] S. Behrem, K. Zarković, N. Eškinja, et al., Distribution pattern of tenascin-C in glioblastoma: correlation with angiogenesis and tumor cell proliferation. Pathology & Oncology Research, 2005 11: 229-35.
[71] D. Si, F. Yin, J. Peng, et al., High Expression of CD44 predicts a poor prognosis in glioblastomas. Cancer Management and Research, 2020, 12: 769-75.
[72] T. Avril, A. Etcheverry, R. Pineau, et al., CD90 expression controls migration and predicts dasatinib response in glioblastoma. Clinical Cancer Research, 2017, 23: 7360-74.
[73] R. Brito, S. Sheth, D. Mukherjea, et al., TRPV1: A potential drug target for treating various diseases. Cells, 2014, 3: 517-45.
[74] M.F. McCarty, J.J. DiNicolantonio, J.H. O'Keefe, Capsaicin may have important potential for promoting vascular and metabolic health. Open Heart, 2015, 2: e000262.
[75] S. Chouaib, Y. Messai, S. Couve, et al., Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Frontiers in Immunology, 2012, 3: 21-21.
[76] H.Y. Kwan, Y. Huang, X. Yao, TRP channels in endothelial function and dysfunction. Biochimica et Biophysica Acta, 2007, 1772: 907-14.
[77] V.P. Kale, S.G. Amin, M.K. Pandey, Targeting ion channels for cancer therapy by repurposing the approved drugs. Biochimica et Biophysica Acta, 2015, 1848: 2747-2755.
[78] L. Leanza, A. Managò, M. Zoratti, et al., Pharmacological targeting of ion channels for cancer therapy: In vivo evidences. Biochimica et Biophysica Acta, 2016,1863: 1385-97.
[79] C.J. Hutchings, P. Colussi, T.G. Clark, Ion channels as therapeutic antibody targets. MAbs, 2019, 11: 265-96.
[80] H. Neumann, Control of glial immune function by neurons. Glia, 2001, 36: 191-9.
[81] W.L. Kong, Y.Y. Peng, B.W. Peng, Modulation of neuroinflammation: role and therapeutic potential of TRPV1 in the neuro-immune axis. Brain, Behavior and Immunity, 2017, 64: 354-66.
[82] J.H. Jeon, Y.J. Choi, I.H. Han, et al., Capsaicin-induced apoptosis in the human glioblastoma U87MG cells via p-38 MAPK and Bcl-2/Bax signaling pathway. Molecular & Cellular Toxicology, 2012, 8: 69-76.
[83] Y.P. Liu, F.X. Dong, X. Chai, et al., Role of autophagy in capsaicin-induced apoptosis in U251 glioma cells. Cellular and Molecular Neurobiology, 2016, 36: 737-43.
[84] J.H. Park, G. Saravanakumar, K. Kim, et al., Targeted delivery of low molecular drugs using chitosan and its derivatives. Advanced Drug Delivery Reviews, 2010, 62: 28-41.
[85] L. Xie, G-H. Xiang, T. Tang, et al., Capsaicin and dihydrocapsaicin induce apoptosis in human glioma cells via ROS and Ca2+‑mediated mitochondrial pathway. Molecular Medicine Reports, 2016, 14: 4198-208.
[86] Y.S. Lee, D.H. Nam, J.A. Kim, Induction of apoptosis by capsaicin in A172 human glioblastoma cells. Cancer Letters, 2000, 161: 121-30.
[87] M. Kaiser, S. Pereira, L. Pohl, et al., Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Scientific Reports, 2015, 5: 10048.
[88] T. Shiobara, T. Usui, J. Han, The reversible increase in tight junction permeability induced by capsaicin is mediated via cofilin-actin cytoskeletal dynamics and decreased level of occludin. PLoS One, 2013, 8: e79954.
[89] M. Kaiser, M. Burek, S. Britz, et al., The influence of capsaicin on the integrity of microvascular endothelial cell monolayers. International Journal of Molecular Sciences, 2008, 20: 122.
[90] M.W. Pitz, A. Desai, S.A. Grossman, Tissue concentration of systemically administered antineoplastic agents in human brain tumors. Journal of Neuro-Oncology, 2011, 104: 629-38.
[91] C. Ferraris, R. Cavalli, P.P. Panciani, et al., Overcoming the blood-brain barrier: successes and challenges in developing nanoparticle-mediated drug delivery systems for the treatment of brain tumours. International Journal of Nanomedicine, 2020, 15: 2999-3022.
[92] S. Beggs, X.J. Liu, C. Kwan, et al., Peripheral nerve injury and TRPV1-expressing primary afferent C-fibers cause opening of the blood-brain barrier. Molecular Pain, 2010, 6: 74.
[93] D-E, Hu, A.S. Easton, P.A. Fraser, TRPV1 activation results in disruption of the blood-brain barrier in the rat. British Journal of Pharmacology, 2005, 146: 576-84.
[94] Z. Jiang, X. Wang, Y. Zhang, et al., Effect of capsaicin-loading nanoparticles on gliomas. Journal of Nanoscience and Nanotechnology, 2015, 15: 9834-9.
[95] A. Carmo, H. Carvalheiro, I. Crespo, et al., Effect of temozolomide on the U-118 glioma cell line. Oncology Letters, 2011, 2: 1165-70.
[96] G.A.J. Lacerda, A.J.A. Díaz, L.S. Pérez, et al., Glioblastoma multiforme del cerebelo. Revista Cubana de Neurología y Neurocirugía, 2012, 2: 141-3.
[97] O.A.J. Moreno, Avances farmacológicos contra el cáncer. Actualidad en farmacología y terapéutica, 2013, 11: 258.
[98] Y. Xin, M. Yin, L. Zhao, et al., Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biology & Medicine, 2017, 14: 228-41.
[99] J.M. Morachis, E.A. Mahmoud, A. Almutairi, Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacological Reviews, 2012, 64: 505-19.
[100] I. Brigger, C. Dubernet, P. Couvreur, Nanoparticles in cancer therapy and diagnosis. Advanced Drug Delivery Reviews, 2002, 54: 631-51.
[101] J Kreuter, Nanoparticulate systems for brain delivery of drugs. Advanced Drug Delivery Reviews, 2001, 47: 65-81.
[102] M. Masserini, Nanoparticles for brain drug delivery. ISRN Biochemistry, 2013, 2013: 238428.
[103] J. Chang, Y. Jallouli, M. Kroubi, et al., Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. International Journal of Pharmaceutics, 2009, 379: 285-92.
[104] H. Xin, X. Jiang, J. Gu, et al., Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials, 2011, 32: 4293-305.
[105] K.J. Lim, S. Bisht, E.E. Bar, et al., A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biology & Therapy, 2011, 11: 464-73.
[106] M.R. Saboktakin, R.M. Tabatabaie, A. Maharramov, et al., Synthesis and characterization of pH-dependent glycol chitosan and dextran sulfate nanoparticles for effective brain cancer treatment. International Journal of Biological Macromolecules, 2011, 49: 747-51.
[107] W. Geldenhuys, D. Wehrung, A. Groshev, et al., Brain-targeted delivery of doxorubicin using glutathione-coated nanoparticles for brain cancers. Pharmaceutical Development and Technology, 2015, 20: 497-506.
[108] L. Yang, S. Gao, S. Asghar, et al., Hyaluronic acid/chitosan nanoparticles for delivery of curcuminoid and its in vitro evaluation in glioma cells. International Journal of Biological Macromolecules, 2015, 72: 1391-401.
[109] C. Fornaguera, A. Dols-Perez, G. Calderó, et al., PLGA nanoparticles prepared by nano-emulsion templating using low-energy methods as efficient nanocarriers for drug delivery across the blood-brain barrier. Journal of Controlled Release, 2015, 211: 134-43.
[110] Y. Cui, M. Zhang, F. Zeng, et al., Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Applied Materials & Interfaces, 2016, 8: 32159-69.
[111] C. Fang, K. Wang, Z.R. Stephen, et al., Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Applied Materials & Interfaces, 2015, 7: 6674-82.
[112] X. Gao, T. Yu, G. Xu, et al., Enhancing the anti-glioma therapy of doxorubicin by honokiol with biodegradable self-assembling micelles through multiple evaluations. Scientific Reports, 2017, 7: 43501.
[113] M. Sack-Zschauer, S. Bader, P. Brenneisen, Cerium oxide nanoparticles as novel tool in glioma treatment: an in vitro study. Journal of Nanomedicine and Nanotechnology, 2017, 8: 474
[114] Y-C. Kuo, C-C. Hsu, Anti-melanotransferrin and apolipoprotein E on doxorubicin-loaded cationic solid lipid nanoparticles for pharmacotherapy of glioblastoma multiforme. Journal of the Taiwan Institute of Chemical Engineers, 2017, 77: 10-20.
[115] Y. Malinovskaya, P. Melnikov, V. Baklaushev, et al., Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. International Journal of Pharmaceutics, 2017, 524: 77-90.
[116] Y. Xu, S. Asghar, L. Yang, et al., Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydrate Polymers, 2017, 157: 419-28.
[117] F.C. Lam, S.W. Morton, J. Wyckoff J, et al., Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nature Communications, 2018, 9: 1991.
[118] Frosina G, Advances in drug delivery to high grade gliomas. Brain Pathology, 2016, 26: 689-700.
[119] N.Y. Hernández-Pedro, E. Rangel-López, R. Magaña-Maldonado, et al., Application of nanoparticles on diagnosis and therapy in gliomas. BioMed Research International, 2013, 2013: 351031.
[120] F.M. Kievit, A. Cooper, S. Jana, et al., Aligned chitosan-polycaprolactone polyblend nanofibers promote the migration of glioblastoma cells. Advanced Healthcare Materials, 2013, 2: 1651-9.
[121] M. Irani, G. Mir Mohamad Sadeghi, I. Haririan, A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. International Journal of Biological Macromolecules, 2017, 97: 744-51.
[122] R.A.A. Muzzarelli, C. Muzzarelli, Chitosan chemistry: relevance to the biomedical sciences: Polysaccharides I: Structure, Characterization and Use. 2005, Springer, Berlin Heidelberg.
[123] M.W. Saltzman, CED of nanoparticles loading with novel agents for improved treatment of gliomas. 2017, Project online: https://projectreporter.nih.gov/project_info_description.cfm?aid =9297226&icde=0YALE University.
[124] M.G. Castro, HDL-loaded nanoparticles for glioma therapeutics. 2016, Project online: https://projectreporter.nih.gov/project_info_description.cfm?aid=9004659&map=y: University of Michigan.
[125] N. Abbott, L. Rönnbäck, E. Hansson, Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience, 2006, 7: 41-53.
[126] L.Y. Ljubimova, Nanoconjugate delivery of proliferation and checkpoint inhibitors to treat glial tumors. 2017, Project online: https://projectreporter.nih.gov/project_info_description.cfm?aid =9266719&icde=0:CEDARS-SINAI Medical Center.
[127] E. Karathanasis, Treatment of glioblastoma using chain-like nanoparticles. 2017, Project online: https://projectreporter.nih.gov/project_info_description.cfm?aid= 9335795&icde=0: Case Western Reserve University.
[128] G.P. Mantellero, C. Gómez-Gaete, C.J. Luengo, et al., Desarrollo de nanopartículas biodegradables de capsaicina y evaluación de cesión del principio activo desde formulaciones semisólidas. Revista de Farmacología de Chile, 2014, 7: 41-9.
[129] N.J. Amruthraj, J.P. Preetam Raj, A. Lebel, Capsaicin-capped silver nanoparticles: its kinetics, characterization and biocompatibility assay. Applied Nanoscience, 2015, 5: 403-9.
[130] F. Xing, G. Cheng, K. Yi, et al., Nanoencapsulation of capsaicin by complex coacervation of gelatin, acacia, and tannins. Journal of Applied Polymer Science, 2005, 96: 2225-9.
[131] A. Sharma, M. Jindal, G.K. Aggarwal, et al., Development of a novel method for fabrication of solid lipid nanoparticles: using high shear homogenization and ultrasonication. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2010, 1: 265-274.
[132] P. Bejrapha. S. Surassmo, M-J. Choi, et al., Studies on the role of gelatin as a cryo- and lyo-protectant in the stability of capsicum oleoresin nanocapsules in gelatin matrix. Journal of Food Engineering, 2011, 105: 320-31.
[133] Y. Zhu, M. Wang, J. Zhang, et al., Improved oral bioavailability of capsaicin via liposomal nanoformulation: preparation, in vitro drug release and pharmacokinetics in rats. Archives of Pharmacal Research, 2015, 38: 512-21.
[134] Y. Zhu, J. Yu, S. Tong, et al., Preparation and in vitro evaluation of povidone-sodium cholate-phospholipid mixed micelles for the solubilization of poorly soluble drugs. Archives of Pharmacal Research, 2010, 33: 911-7.
[135] Y. Zhu, W. Peng, J. Zhang, et al., Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: Preparation, in vitro and in vivo evaluation. Journal of Functional Foods, 2014, 8: 358-66.
[136] W. Peng, X.Y. Jiang, Y. Zhu, et al., Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharmacologica Sinica, 2015, 36: 139-48.
[137] L.M. Cirino, D.M. Vergne, P.F. Santana, et al., Decreased inflammatory response in rat bladder after intravesical administration of capsaicin-loaded liposomes. Annals of the Brazilian Academy of Sciences, 2016, 88: 1539-47.
[138] F.M. Goycoolea, A. Valle-Gallego, R. Stefani R, et al., Chitosan-based nanocapsules: physical characterization, stability in biological media and capsaicin encapsulation. Colloid and Polymer Science, 2012, 290: 1423-34.
[139] T.K. Giri, P. Mukherjee, T.K. Barman, et al., Nano-encapsulation of capsaicin on lipid vesicle and evaluation of their hepatocellular protective effect. International Journal of Biological Macromolecules, 2016, 88: 236-43.
[140] E. Martínez-Benavidez, S.E. Herrera-Rodríguez, O.Y. Lugo-Melchor, et al., Nanocápsulas de capsaicina y su actividad antitumoral en células de glioblastoma humano. Revista Salud Jalisco, 2020, 7: 96-102.
Copyright© Evelin Martínez-Benavidez, Inocencio Higuera-Ciapara, Sara Elisa Herrera-Rodríguez, Ofelia Yadira Lugo-Melchor, Francisco Martín Goycoolea, and Francisco Javier Guerrero Jazo. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.