Review
Therapeutic Nanoparticles: Recent Developments and Their Targeted Delivery Applications
Mahendra Kumar 1 *, Umesh Kumar 1, Alak Kumar Singh 1
1Harcourt Butler Technical University, Kanpur-208002, India
* Corresponding author. E-mail: mahendra.bbt@gmail.com
Received: Mar. 17, 2021; Accepted: Jun. 11, 2021; Published: Apr. 23, 2022
Citation: Mahendra Kumar, Umesh Kumar, and Alak Kumar Singh, Therapeutic Nanoparticles: Recent Developments and Their Targeted Delivery Applications. Nano Biomed. Eng., 2022, 14(1): 38-52.
DOI: 10.5101/nbe.v14i1.p38-52.
Abstract
The effective delivery of drugs to the targeted tissues or cells has always been a cause of concern. Nanotechnology has emerged as an effective tool to solve this problem of targeted drug delivery. The development of nanoparticle drug delivery systems is a revolutionary step in the healthcare domain. Nanoparticles loaded with drugs, known as nanomedicines, are used to achieve site-specific delivery of drugs that reduces the amount of required dose and, hence, toxicity. The nanoparticles, due to their small sizes, can easily cross the cell barriers. Also, the surface of the nanoparticles can be modified in such a way that it can be recognized by the molecules on the targeted cells. The addition of ligands, antibodies, aptamers, etc., is done to modify the surface. Therefore, these nanoparticle drug delivery systems are used to obtain targeted delivery of drugs, controlled delivery, biocompatibility, low toxicity and degradation within acceptable time period. The novel drug delivery techniques involving nanoparticles are designed to improve the pharmacological and therapeutic properties of drugs. Nowadays, metallic as well as biodegradable nanoparticles are used as effective drug carriers for cancer, cardiovascular diseases, brain related disorders and so on. Metallic nanoparticles are obtained by the reduction of metallic ions from their solutions to the nano-size range. Of the metallic nanoparticles, Gold is studied very extensively due to its inert nature and relatively high biocompatibility than other metals. Biodegradable nanoparticles are synthesized from polymeric substances such as polylactic acid (PLA), gelatin, chitosan, etc. Some other examples of nanoparticles are dendrimers, liposomes, carbon-based, viral based, etc.
Keywords: Nanotechnology, Nanomedicines, Silver Nanoparticles, Drug Delivery, Site specific Delivery.
Introduction
Nanotechnology is the study and application of extremely small things and can be used across manipulation, production, control and utilization of materials going in nanometres The knowledge of nano-science is dedicated in creation of useful revolutionary products that have a huge impact on all forms of life. It refers to the study of interactions between molecular, cellular, and engineered materials at an incredibly small size-range. Nanotechnology impacted various industries such as Electronics, Textiles, Biomedical Industry and many more. [5] Recent developments in the field of nanomaterials have led to their applications in medical fields also. The confluence of technology with biology has made it possible to develop advanced medical treatments and medicines. Nanomedicines are developed in order to increase the efficiency of treatment of various devastating diseases known to man, including cancers [12, 91]. The main reason why nanoparticles are now being used so widely is because the properties of such small particles are not available in distinct molecules and bulk materials [53, 88]. Advances in nanobiotechnology in recent years have led to the development of newer concepts of drug delivery to the targeted organs [25, 52]. It focuses on the development of nanocarriers such as micelles, nanobots, metallic nanoparticles, liposomes and other polymeric nanoparticles which have greater advantages in drug delivery. Nanomedicines help in increasing the interaction of drug with the target tissues and cells and also protects it from degradation before its action. It offers a considerable means of delivering small molecules such as peptides, nucleic acids, and other micro as well as macro molecules either by localized or targeted delivery [53, 66]. The nanocarriers have high specificity towards the targeted organ and at the same time it reduces the toxic effects of the drug [98]. The nanocarriers have a great deal of advantage when compared to traditional drug targeting. Once, these nanoparticles /carriers are conjugated with specific affinity ligands such as peptides and small molecules, these can be used to detect molecular biomarkers and tumour cells with high sensitivity [70]. The nano size of the delivery vehicle provides distinct advantages over any other solution, due to which they can easily pass through different barriers present inside the cells, effectively delivering the drug molecules to its target cells. The bi-conjugated ligands on the surfaces of the nanoparticles direct them to the target organs/cells and once they reach there, they are effectively taken up by the cells through receptor mediated process [51]. The methods for the administration of the drug can be divided into: Invasive method and non-invasive method. The non-invasive method involves delivering of drugs through oral and nasal passages while invasive methods include injection of nanoparticles through a vein. Each method has its own advantages as well as drawbacks [98]. The success of these particles is largely dependent upon the characteristics of drugs as well as the nanoparticle itself, i.e., its solubility, stability and also on the ability of the nanoparticles to overcome the biological barriers in reaching the target. The main hurdle in reaching the target tissue also depends upon the nature of the drug being transported. Although, functionalized nanoparticles have been developed by scientists over years with greater efficiency, however, these nanocarriers often face certain limitations such as poor drug loading, size differences, low stability of the drug in vivo, etc. [34]. Noble metals such as Cu, Au, Ag, and Pt are promising drug delivery particles [40]. The functionalized metal nanoparticles have greater advantages in targeted and controlled release of the drug molecules [70]. Silver nanoparticles are the most promising nanocarriers as it does not affect the living cells. The metallic nanoparticles are advantageous over any other nanocarrier as they have high density surface ligand attachment, trans-membrane delivery [28], protection of the attached medicine from degradation and potential for timed release of the therapeutics [78, 24]. The photophysical properties of inert metal nanoparticles have enabled these materials to be used commonly as drug delivery devices enabling targeted delivery and delivery confirmation via imaging. [37]
Ttpes of nanoparticles
The nanoparticles exist in various forms, shapes and sizes [13]. Their properties and structures determine their application for a process, especially the medical applications. The nanoparticles are most extensively studied for drug delivery purposes. The nanoparticles that are used for drug delivery are of two types: (a) Organic nanoparticles and (b) Inorganic nanoparticles. The organic polymers include polymeric nanoparticles, dendrimers, liposomes and solid lipid nanoparticles while the inorganic nanoparticles include metal nanoparticles (gold, silver, platinum, quantum dots, etc.) and silica nanoparticles [76]. These nanoparticles have properties of their own which makes them suitable for various drug delivery systems. Therefore, these nanoparticles have to be carefully chosen, so that, it is possible to carry out the process in the required manner. There are various types of nanoparticles that are used in drug delivery. Some of them are described in the following paragraphs.
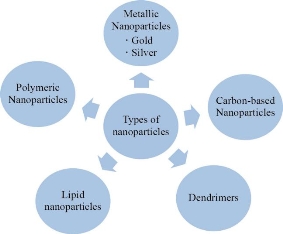
Fig. 1 Types of nanoparticles.
Metallic nanoparticles
The metallic nanoparticles are the ones that are made from metals. These are formed by reduction of the ions of the metal from its aqueous solution leading to the synthesis of reduced particles in the range of nanometers. These metallic nanoparticles are then used for a variety of purposes after certain modifications [70]. These metallic nanoparticles can be used in diagnostics as well as drug delivery [46]. Metallic nanoparticles increase therapeutic index of drug, its half-life and also have the potential to carry heavy doses of drugs [61]. The most widely used nanoparticles in drug delivery are gold nanoparticles.
Gold nanoparticles
The application of gold nanoparticles in the medical field is not new but it is used for the treatment of diseases from centuries ago. Sastry et.al., have reported the extracellular synthesis of gold nanoparticles by fungus Fusarium oxysporum and Actinomycetes (Thermomonospora sp.) [58, 3] The intracellular synthesis of gold nanoparticles by fungus Verticillium species was reported as well [56]. The gold nanoparticles are most widely used for the diagnosis of cancer [32]. Gold nanoparticles with different morphologies can also be synthesized in human cancer and non-cancer cells, assisting in the diagnosis [70, 7].
Silver nanoparticles
These nanoparticles have antimicrobial property against Gram-negative and Gram-positive bacteria [65]. It was observed that Pseudomonas stutzeri AG259, isolated from silver mines, reduced silver nitrate solution to silver nanoparticles in its periplasmic space. [75] The silver nanoparticles can be synthesized biologically by bacteria or fungi. Silver nanoparticles, with some modifications on its surface, can be used for the purpose of drug delivery because it is inert and causes less toxic effects.
Polymeric nanoparticles
Polymeric nanoparticles are colloidal nanoparticles containing the therapeutic agent either encapsulated or adsorbed on the surface of the nanoparticle [15]. These particles have the advantage of better circulation within the fine blood capillaries without getting agglomerated, thus, preventing blockage, and also the intracellular uptake rate of these nanoparticles is much higher as compared to particles of even slightly bigger size [66]. Not only this, but nanomedicines also helps to enhance the interaction of the drug with the tissues or cells, protects it from getting degraded prior to completion of its action, increase its adsorption into a tissue and maintain the required pace for the release of drug. The polymeric nanoparticles used in drug delivery can be synthetic, natural (e.g. chitosan, gelatin, proteins, etc.) or pseudo synthetic (synthetic polypeptides) [82, 23]. The polymeric nanoparticles are biocompatible and biodegradable. They have stable structure and their drug holding and release characteristics can be easily modified by changing the type of polymer used, its length, and method of synthesis. Surface modifications, such as attachment of ligands, also allow for targeted delivery of the drug to the required tissue or cell [4, 85]. Protein and peptide drugs can be conjugated with polymeric nanoparticles such as PEG to prevent it from getting degraded inside the body prior to its action, thereby, increasing its half-life [98]. The polymeric nanoparticles provide a lot of advantages in terms of drug loading, release, biodistribution, provide stability to drugs and increase their solubility, protect drug from degradation and also enhance targeted delivery of drugs [61].
Lipid nanoparticles
Nanoliposomes are lipid vesicles, spherical in shape, having a membrane bilayer that is made up of lipid molecules which are amphiphilic in nature. These nanoliposomes can be synthetic or natural. The most distinguishing feature of nanoliposomes is that it does not require any modification to carry either hydrophilic or hydrophobic drugs [85]. The drug to be delivered can be encapsulated within the vesicles or between the lipid bilayer, depending on its nature. [54] These particles are used in the treatment of infections, meningitis and cancer. [61] The liposomes are biocompatible, biodegradable, low toxicity, high efficiency, increases drug solubility, improves pharmacokinetic properties and therapeutic indices of the drugs. Its surface charge, shape and size can be easily modified for targeting a drug to a particular site. The disadvantages are low encapsulation efficiency, poor holding capability and quick release of drugs. Nanoliposomes can be coated with PEG to suppress its degradation by macrophages [98]. Solid lipid nanoparticles are the ones made from solid lipids having an average diameter between 50-1000 nm. These particles can be effectively used to deliver drugs. It includes colloidal lipid particles that remain solid at room temperature [6]. It has advantages with respect to drug incorporation in the particle but loading capacity is low [61]. It can be most effectively used for parenteral applications [59].
Dendrimers
Dendrimers are macromolecules having an ordered and highly branched structure having a core, branches coming out from core, and functional groups attached on it [61, 85] The branched structure provides high ratio of surface area to size [8]. The properties of dendrimers, for example, its shape, size density, surface functionality, etc., can be controlled and modified. Dendrimers have anti-tumour, antiviral and antibacterial activities [31]. These are biocompatible and generally regarded as safe for the process of drug delivery [19].
Carbon-based nanoparticles
The carbon based nanoparticles consist of C60 fullerenes, carbon dots, nano-diamonds and nanofoams. Carbon nanotubes can be single layered or multi-layered graphite having a tubular arrangement [19]. The carbon based polymers have to be modified to make them biocompatible and are, hence, coated with PEG or hyaluronic acid matrix. The drugs can either be encapsulated or surface adsorbed. The drug released can be controlled electrically or chemically [9]. The major problem with carbon based nanoparticles is toxicity. These particles may induce inflammatory responses and granuloma formation due to overstimulation of the complement system [61, 77].
Synthesis of Nanoparticles
There are different methods involved in the synthesis of nanoparticles. The conventional methods of synthesis have a wide range of limitations such as - these are expensive processes, they are responsible for the generation of various hazardous chemicals, etc. Hence, owing to these limitations, researchers have found a way to develop these particles in a safe and eco-friendly manner. Among the new found processes, biological processes are most focussed ones that exploited for the green synthesis of nanoparticles. The biological methods of nanoparticle synthesis yields nanoparticles of precise shape and size and, therefore, these methods are most widely accepted for the synthesis of desired nanoparticles [39]. The common methods involved in the synthesis of silver nanoparticle.
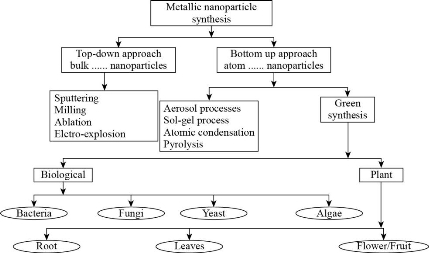
Fig. 2 Various approaches for metallic nanoparticle synthesis.
Chemical synthesis
Several chemical methods have been used for the synthesis of silver nanoparticles. These processes are mostly employed for quick production of nanoparticles. SNPs can be produced by the use of reducing agents such as sodium hydroxide, HCHO, Sodium bicarbonate, etc. Silver sols production was reported in 1979 by reduction of silver nitrate in solution of NaBH4 (in excess). This solution was mixed quickly to yield nanoparticles in the size range of 1-50 nm. Another method includes the use of formaldehyde as a reducing agent for preparation of SNPs. In this process PVP (Polyvinylpyrrolidone) is added to silver nitrate and the mixture is added to formaldehyde sol to yield SNPs of 7-20 nm size [14]. Other chemical methods are also high yielding processes commonly used at laboratory and industrial scales for production of silver nanoparticles including sol gel method [70], chemical vapour synthesis (CVS), etc. [1].
Green synthesis
Green synthesis includes biological synthesis and plant synthesis.
Biological synthesis
Due to the increased environmental and health risks associated with the synthesis of nanoparticles many researchers have developed different ways to synthesize nanoparticles. Out of all the methods currently employed in the synthesis of NPs biological synthesis are the most promising methods for green synthesis of nanoparticles [57]. Biologically, the synthesis of nanoparticles can be brought about by unicellular or multicellular organisms which produce nanoparticles, either intracellularly or extracellularly [83, 63]. Biological synthesis includes synthesis of nanoparticles with bacteria, fungi and other microorganisms.
Bacterial synthesis
The synthesis of nanoparticles have been reported with many bacterial strains such as that of Lactobacillus, Pseudomonas, etc. Bacteria are the most commonly found prokaryotic organisms that are capable of synthesizing nanoparticles. In the year 1999, Klaus and co-workers reported the biological synthesis of silver nanoparticles by the use of bacterial species of Pseudomonas that synthesized the nanoparticles of diameter 200 nm within its periplasmic space [41, 42]. This strain was isolated from silver mine and the cells of these bacteria were cultured in the presence of high concentration of silver nitrate, after which, the silver nanoparticles were isolated using various methods. There are many other methods of synthesizing silver nanoparticles including processes such as plasma spray method [38], high pressure spurting [12], genetically engineered human l subunit ferritin, etc. [44]. It was found that the Lactobacillus species that was found in buttermilk produced intracellular nanoparticles of silver, gold and their alloy in their aqueous solution. The cells of Lactobacillus was found to have retained their viability even after the accumulation of nanoparticles [62].
Fungal synthesis
Fungi are the organisms that offer a large number of advantages when it comes to biological synthesis of nanoparticles. The fungi are hence, most widely studied organisms for the synthesis of nanoparticles with the advantage of having high tolerance to metals as well as high bioaccumulation capacity of the nanoparticles and are easier to grow with no difficulty in handling [92]. Several fungal strains such as Fusarium solani are known to synthesise silver nanoparticles [33]. They were grown aerobically in a liquid medium containing various nutrients such as glucose, 10.0 g/L, yeast extract, 0.6 g/L, Ammonium sulphate (NH2)SO4, 1.0 g/L, KH2PO4 (7.0 g/L); MgSO4·7H2O, (0.1 g/L). The flasks containing the media were inoculated with the fungal spores and then incubated at a temperature of 28 °C and agitated at 120 rpm for 72 h. The biomass was grown which, after repeated washing with distilled water followed by agitation at 120 rpm, yielded silver nanoparticles [21]. Silver nitrate was added to the cell free culture, and after addition of silver nitrate to the mixture colour changes from colourless to brown whose intensity increases with time showing the reduction of silver nitrate by Fusarium solani [22]. It was reported that the species of Aspergillus fumigatus could produce silver nanoparticles extracellularly, when exposed to silver ion in solution [11]. As reported by N. Vigneshawaram, Aspergillus flavus produced silver nanoparticles in silver nitrate solution. These particles accumulated on the surface of the cell wall of the fungi. These particles gave absorption peak at 420 nm when observed in UV-Vis spectrophotometer [96]. Mukherjee et.al. used Verticillium for the production of silver nanoparticles by exposing the fungi with silver ions that led to the intracellular accumulation of silver nanoparticles [57]. Fusarium oxysporum is also used for the synthesis of silver nanoparticles. The aqueous silver ions are reduced by Fusarium oxysporum to hydrosols of silver nanoparticles with size of silver nanoparticles in the range of 5-15 nm which are extremely stable due to proteins secreted by the fungi in solution [2]. Sadowski et.al. have used Penicillium isolated from soil to produce extracellular silver nanoparticles [75]. It is believed that the reductases produced by the fungi are responsible for the reduction of silver ions from its solution leading to the formation of silver nanoparticles [92].
Yeast in nanoparticle synthesis
Kowshik et.al, have reported the synthesis of silver nanoparticles with MKY3 which is a silver tolerant species. When MKY3 is exposed to silver solution in its log phase of growth, it precipitates silver ions as nanoparticles in the size range of 2-5 nm [43].
Plant synthesis
Different resources can be utilised for the synthesis of nanoparticles. Since ancient times, plants are known to possess various compounds with great therapeutic values. Recent discoveries have led the scientists to synthesize nanoparticles using plants. The major advantage of using plants as a source of nanoparticles is that these are easily available, less expensive and nontoxic. [39]Many plant species have been studied for the synthesis of nanoparticles. Plants have biomolecules like coenzyme with commendable potential to diminish metal salt into nanoparticles. Like other biosynthesis cycles, gold and silver metal nanoparticles were first examined in plant . Different plants [including aloe vera (Aloe barbadensis Miller), Oat (Avena sativa), horse feed (Medicago sativa), Tulsi (Osimum sanctum), Lemon (Citrus limon), Coriander (Coriandrum sativum), Mustard (Brassica juncea) and lemon grass (Cymbopogon fexuosus)] have been used to synthesize silver nanoparticles with gold nanoparticles too.[88,89,104] It was reported that Neem (Azadirachta indica) leaf broth with aqueous solution of silver nitrate could lead to the production of pure silver nanoparticles extracellularly [79, 80]. The time required for the reduction of silver ions was 4 hrs and for gold ions was 2 h. This is comparatively very less in comparison to that of bacteria that takes 24 h and fungi that requires 120 h for the reduction of silver and gold ions to nanoparticles. Also, the stability nanoparticles from leaf broths was higher due to release of surface active agents in leaf broth that stabilize the nanoparticle suspension [75].
Characterization of Nanoparticles
It is important to determine the properties of materials or substances for using it for a defined purpose. The use of nanoparticles in drug delivery is a critical process and hence, it is very important to know all the characteristics of the nanoparticles before putting it to use. The physical and chemical properties have to be determined because they are responsible for the biosafety, bio-distribution, drug loading and delivery, efficacy and other functional aspects such as shape, size, surface charge, morphology, topology, hydrophilic or hydrophobic nature, etc. Density, molecular weight and crystallinity determine the release of drug and its degradation, while hydrophobic nature affects the behaviour and interaction of nanoparticles within the body [23]. The various techniques used to characterize nanoparticles are as follows.
UV-Vis spectroscopy
UV-VIS spectroscopy is one of the simplest techniques used for the primary characterization of nanoparticles [101, 35]. Based on Beer- Lambert’s law, it is a technique used for the detecting the presence or absence of nanoparticles in a colloidal solution. Although, this technique does not give detailed information regarding morphology of nanoparticles, yet it gives an accurate result regarding the size distribution of nanoparticles in the solution. Thus, this method is used for semi-quantitative analysis of the nanoparticles [70]. This is a very reliable method that has the advantages of being fast, simple, sensitive and no prior calibration is required for the analysis of the nanoparticles [93]. The free electrons in the silver nanoparticles absorb light of a particular wavelength and give the absorption peak at a particular wavelength. The breadth of chromatogram peak will be indicative of the size distribution of nanoparticles in the solution but to confirm the morphology, other techniques for imaging should be used [70]. Different studies have shown the absorbance of nanoparticles with sizes ranging from 2-100 nm with a light of wavelength of 200-800 nm [74]. As for the nanoparticles obtained by the reduction of silver nitrate with Aspergillus flavus, Vigneshwaran et.al., observed the spectra from 200-800 nm at an interval of 24 h. The nanoparticles showed absorption at 420 nm [96]. In another study involving Fusarium solani, the absorption peak was observed at 415 nm after 72h of incubation [21].
Dynamic light scattering (DLS)
This is the most commonly used technique for the analysis of nanoparticles [101]. DLS is a technique used to study the size distribution of the small particles (2-500 nm) present in a colloidal solution [93]. It determines the physicochemical properties of nanoparticles. It is based on interaction of light with particles such as changes in light field. The suspended solutes in a solution have a property of scattering light, based on Rayleigh Scattering, that is passing through it and this is what the DLS study analyses [26]. The DLS measures the diffusion coefficient for solutes which is dependent on the hydrodynamic radius of the molecules. Thus, the DLS gives information about the hydrodynamic radius and therefore, average diameter of nanoparticles, their polydispersity indices as well as zeta potential [70, 36]. The result is somewhat influenced due to the presence of Brownian motion, otherwise, it is an extremely precise technique [101].
Scanning electron microscopy (SEM)
This is the imaging technique used for the analysis of nanoparticles by using a focused beam of highly energetic electrons [70]. The high energy electrons are responsible for generating a variety of signals at the surface of solid samples. SEM is a high resolution microscopy technique that enables the study of surface morphology of the particles with great precision, giving information about the particle size, size distribution, shape, chemical composition and crystalline structure of the particles at nanoscale. SEM cannot give information about the internal structure of the particles but gives the purity of the sample [101].
Transmission electron microscopy (TEM)
TEM is an analytical tool that is used to study the morphological characteristics and arrangements of the nanoparticles [70]. It provides a high resolution image of the nanoparticles and gives a quantitative measure of the particle size, polydispersity as well as morphology [49, 100]. It gives better and more precise information regarding the spatial resolution of the nanoparticles as compared to SEM [74]. The preparation of sample is a tedious task but important to get good results [101]. A. Ahmad et al. studied the silver nanoparticles with TEM and in the image, they observed individual silver nanoparticles along with their aggregates with spherical and, less commonly, triangular morphologies. The size of silver nanoparticles in the aggregates was between 5-50 nm [2, 95].
Atomic force microscopy (AFM)
AFM is an analytical tool that is widely used to study the nanoparticles and its imaging [27]. It is used to analyse the dispersion and aggregation of nanoparticles. Also, the size, structure, shape and other topographical features can be determined by AFM. It is also used to investigate the materials doped with nanoparticles [68]. The advantages of AFM include high speed and resolution, quick and easy preparation of sample, no special requirement of surface as being oxide-free or electrically conductive and it does not damage the surface [18, 70]. Apart from some errors in estimation of size due to size of cantilever, it gives a good result if proper steps are taken [30, 101].
X-ray diffraction (XRD)
X-ray diffraction is a technique that is used to analyse crystal structures along with molecular structures of various compounds [99, 17]. It is based on Bragg’s Law. X-ray incident on a crystal gets diffracted and forms a pattern that shows the crystal structure, morphology, nature, etc. [101]. It is now used for the characterization of nanoparticles and materials made out of it [16]. It can also be used to determine the purity of samples. A study showed the XRD for silver nanoparticles embedded in fungal mycelia to have diffraction intensities from 30° to 80° 2Ɵ angles [91, 96].
Fourier transform infrared spectroscopy (FTIR)
The IR spectra gave an idea about the nearby sub-atomic status of the organic molecules on the surface of nanoparticles. FTIR spectral measurements were done to recognize the potential biomolecules which are answerable for reducing and capping the bio-reduced nanoparticles. Fourier transform infrared spectroscopy (FTIR) is a strategy that is utilized to examine the chemistry of numerous natural synthetics, polymers, paints, coatings, oils etc. FTIR analysis can give not only qualitative (identification) analysis of materials, but, with relevant standards, can be used for quantitative (amount) analysis [51, 98].
Zeta potential
Zeta potential assurance is a huge portrayal strategy of nanocrystals to appraise the surface charge, which can be utilized for understanding the physical stability of nanosuspensions . A huge positive or negative worth of zeta capability of nanocrystals demonstrate great physical stability of nanosuspensions because of electrostatic aversion of individual particles. A zeta potential worth other than −30 mV to +30 mV is for the most part considered to have adequate shocking power to achieve better actual colloidal stability [107]. Then again, a little zeta potential worth can bring about molecule accumulation and flocculation because of the van der Waals attractive forces follow up on them. These might bring about physical instability.
Drug Loading
Drug loading refers to the synthesis of nanoparticles that are polymeric (not necessarily) colloidal particles containing the therapeutic agent either encapsulated in the polymeric capsules (nanocapsules, reservoir-type nanodevices) or conjugated at the surface of the nanoparticle (nanospheres, matrix-type nanodevices) or adsorbed on the surface [66, 15]. The nanoparticles that are used as drug delivery vehicles should have certain characteristics to be used as a successful drug carrier. It should be inert and should not contain impurities that can leach into the body along with the drug. It should also be biodegradable [87]. Another important consideration is the loading capacity of the nanoparticles [45]. The nanoparticle having higher drug loading capacity is a better delivery vehicle as lesser amount of dose of the nanoparticles would be required to deliver the intended quantity of the drug [89]. Drug loading onto nanoparticles can be done in two ways- first, by incorporating the drug into the nanoparticle at the time of its synthesis, called nanoencapsulation. Second, by incubating the nanoparticles in the drug solution leading to its surface adsorption. Thus, drug loading can be achieved by adding the drugs to a solution that contains preformed nanoparticles or to the reaction mixture during the formation of nanoparticles [84].
The drug loaded nanoparticles can be separated from the solution containing loose drug molecules by ultracentrifugation of gel filtration. The encapsulation efficiency (EE), the efficiency of nanoparticles to encapsulate the drug, can be given as follows:
EE = Amount of bound drug / Total amount of drug used at the time of nanoparticle synthesis [90].
It can also be obtained by subtracting the amount of drug remaining in the supernatant from the total amount of drug that was added in the solution. Thus, the chemical nature of drug, chemical structure of polymer as well as drug loading method influence the amount of drug bound to the nanoparticles and its interaction with them [81].
Preparation of Nanoparticles Containing Drugs
The drug containing nanoparticles can be prepared by following methods: (a) Dispersion of preformed polymers, (b) polymerization of monomers, and (C) ionic gelation for hydrophilic polymers. [50]
Dispersion of preformed polymers
The nanoparticles obtained by this method are polylactic acid (PLA), poly(β-hydroxybutyrate) (PHB), poly lactic-co-glycolic acid (PLGA) and poly(ε-caprolactone) (PCL). This technique can be done by the following methods.
Solvent evaporation method
In this method, an organic solvent, such as dichloromethane or chloroform, containing dissolved polymer is emulsified with drug to form oil/water emulsion having some surfactants, example, gelatin or polyvinyl alcohol [50]. The solvent is evaporated by subjecting it to high temperature or pressure with continuous stirring, once stable emulsion is formed [72]. Water soluble drug loaded nanoparticles can be prepared by using W/O/W method [89]. The stirring speed, concentration of polymer used and type of stabilizer affects the nanoparticles formed [50].
Spontaneous emulsification / solvent diffusion method
In this method, a water miscible solvent is used together with an organic solvent that is insoluble in water to produce oil-water droplets. Turbulence is created at the interface between the two phases because of spontaneous diffusion of solvents out of the droplets. This leads to the formation of nanoparticles by condensation. The size of nanoparticle obtained is dependent in the concentration of water soluble solvent and reduces with increasing concentration [98, 50]. The process is simple, highly reproducible, easy to scale-up and has narrow size distribution [69].
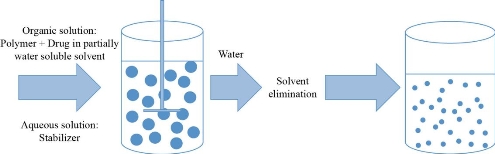
Fig 3 Solvent diffusion method. Adapted from C.P. Reis, R.J. Neufeld, A.J. Ribeiro, et al., Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2006, 2(1): 8-21.
Nanoprecipitation method
Drugs and polymers are dissolved in polar and water miscible solvents such as ethanol, methanol or acetone. Then, it is poured into an aqueous solution, containing surfactant, drop wise. This forms nanoparticles due to rapid solvent diffusion. Then, the nanoparticles can be recovered by removing solvent under low pressure. This method is most commonly used for encapsulation of hydrophobic drugs but can also be hydrophilic drugs also [50].
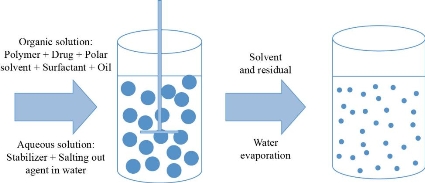
Fig. 4 Nanoprecipitation. Adapted from C.P. Reis, R.J. Neufeld, A.J. Ribeiro, et al., Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2006, 2(1): 8-21.
Salting out method
Water miscible organic solvent containing dissolved polymer is emulsified with an aqueous phase with the use of strong mechanical forces. High concentration of salts which are insoluble in organic solvent are added in the aqueous phase containing emulsifier. In this method, solvent does not diffuse due to salting out agents (electrolytes such as magnesium chloride hexahydrate or magnesium acetate tetrahydrate, calcium chloride or non-electrolytes such as sucrose). When pure water is added to the mixture with continuous stirring, water soluble organic phase forms nanoparticles which can be recovered by cross flow filtration or centrifugation [72, 50].
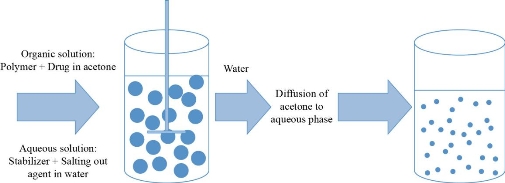
Fig 5 Salting out method (Adapted from C.P. Reis, R.J. Neufeld, A.J. Ribeiro, et al., Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 2006, 2(1): 8-21.
Polymerization methods
Polymerization can be used to synthesize nanoparticles from its monomers. The nanoparticles of poly-butyl-cyanoacrylate or poly-alkyl-cyanoacrylate can be formed by this method. The drug can be added to the solution before adding monomers or after the completion of the polymer formation to get it adsorbed on the surface of nanoparticles. The nanoparticles can be obtained from the solution by ultracentrifugation [89, 69]. The size of nanoparticles is determined by the concentration of surfactant and stabilizers added [67].
Ionic gelation method for hydrophilic polymers
The nanoparticles of natural polymers such as gelatin, alginate or chitosan can be obtained by this method. These are hydrophilic and biodegradable. This method involves the conversion of liquid materials into gels at room temperature because of the ionic interactions. [50] For example, the emulsified gelatin droplets are hardened by cooling below the gelation temperature in ice bath to obtain gelatin nanoparticles [94].
Surface Modifications
The properties that influence the targeting capacity of drug and its retention time in the body are particle size, charge, surface modifications and hydrophobicity. Surface charge is an important factor that determines the interaction of nanoparticles with blood cells or oppositely charged membrane cells. Cationic charge increases the interaction of nanoparticles with the cells and thus, promotes internalization. The hydrophobic surface charge allows the nanoparticles to be opsonized and thus, cleared by the phagocytic system of the body. To prevent this, the nanoparticles are modified to create hydrophilic surface by coating them with hydrophilic polymers such as polyethylene glycol which repel the plasma proteins and, therefore, increase the circulation time in blood [89]. These nanoparticles containing drugs are called “long-circulation” or “PEGylated” nanoparticles are able to target the tissues passively due to enhanced permeability and retention time (EPR) [15]. PEG can be added by different methods; for example, while using poly dopamine nanoparticles for combined chemo- and photo-thermal therapy for cancer, Michael addition reaction was used to PEGylate the poly dopamine nanoparticles [102]. The molecular weight of the polymer affects the release mechanism of the drug. The higher the molecular weight of the polymer used for the encapsulation of drug, the slower will be the release [89].
Drug Release
The release of drug from the nanoparticles and its biodegradation are important considerations while developing an effective drug delivery system. The drug release can be either sustained or stimuli-responsive. The sustained delivery systems deliver drugs at a specific rate over a period of time [90]. The stimuli-responsive drug delivery systems can be endogenous as well as exogenous. The exogenous systems of drug delivery use the external stimuli to release the drugs. Such systems have one or more components that are responsive to some specific stimuli, for example, temperature changes, magnetic fields, electric fields, ultrasound and light. The endogenous stimuli responsive systems, on the other hand, make use of internal factors of the organs or tissues to release the drugs into the environment. For example, redox potential, pH and concentrations of specific molecules [60]. The rate of drug release depends on the following factors: (1) Desorption of the surface-bound/adsorbed drug; (2) drug diffusion through the nanoparticle matrix; (3) diffusion (in case of nanocapsules) through the polymer wall; (4) nanoparticle matrix degradation; and (5) a combined erosion or diffusion process [73]. Release of drugs from the polymer depends on the diffusion, biodegradability and solubility [84]. The release of drug also depends on the size of nanoparticles and encapsulation efficiency. The surface area to volume ratio is high for smaller particles that keeps most of the drug particles at or near the surface, this allows their release at a faster rate. Larger particles can encapsulate more drug but release is slower [71]. The rate can be modified by taking the suitable polymeric material as the carrier. In case of nanospheres, the rate of drug release occurs by the process of diffusion or matrix erosion [84]. The burst release of drug can be attributed to larger particle size or improper attachment of the drug with the nanoparticle [50]. To study the in vitro release of drug from the nanoparticles many techniques are used. Some of these techniques include: (1) Reverse dialysis sac technique, (2) side-by-side diffusion cells with artificial or biological membrane, (3) dialysis bag diffusion technique, (4) ultracentrifugation, (5) ultrafiltration, and (6) centrifugal ultracentrifugation techniques [89].
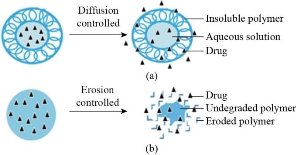
Fig. 6 Sustained drug delivery systems. (a) Diffusion controlled release of drug; (b) erosion controlled release of drug. Adapted from T. Sun, Y.S. Zhang, B. Pang, et al., Engineered nanoparticles for drug delivery in cancer therapy. Angewandte Chemie International Edition, 2014, 53(46): 12320-12364.
Site Specific Delivery
The delivery of drugs with the help of nanoparticles have proved to be a major development in the field of medicine. This is due to the fact that nanoparticles offer the advantage of targeted drug delivery. Earlier, when nanoparticles were not put to use in the field of medicine, it was difficult to achieve drug delivery to the specific site. The drug was administered into the body and it had to travel in the whole body because of which not only the disease affected cell, tissue or organ was exposed to it but also the normal ones. This has enhanced toxic effect on the body. With the application of nanoparticles, targeted delivery can be achieved to minimize toxicity as well as reduce the required dose of the drug. With the help of surface modifications such as addition of ligands for specific receptors, antibodies, protein, peptides, charged molecules, etc., highly specific site delivery can be achieved [8]. It is also seen that in case of PLGA nanoparticles that are embedded inside a hydrogel, the release of protein molecules is extended and initial burst is reduced because hydrogels offer localization of these nanoparticles at their site of injection [64]. Site specific delivery can be done in two ways: Active and passive. The active drug targeting specific ligands or antibodies specific to the receptors that are expressed by the affected cells, such as tumour cells, are attached on the drug loaded nanoparticles. These ligands are recognized by the specific cells only leading to targeted delivery of drug and the nanoparticles can get endocytosed in the cell where the release of drug occurs due to change in pH or enzymatic conditions [47, 98]. It also has the advantage of having concentrated delivery of drug that causes less toxicity and decreased dose requirement [55]. The passive drug targeting strategy involves the entry of drug-carrier complex into the cell through the phagocytic system through macrophages [55]. The drugs enter the cells due to enhancement in its permeability [98]. The degradation of carrier leads to diffusion of drug into the cell. By controlling the size of the nanoparticle-drug conjugate, its diffusion to other non-target sites can be prevented and targeted delivery is achieved [55].
Future Prospects
Nanoparticles are highly advantageous when it comes to the targeted delivery of drugs and release. The major concerns are to improve the bioavailability, stability, drug loading capacity and release mechanisms. The toxicity caused by nanoparticles and their effective degradation and removal from the body are yet to be fully understood and determined [86, 20]. Also, the pharmacokinetic properties have to be taken under consideration while developing the nanomedicines. It is important to develop imaging systems for the nanoparticles to be monitored for their activity inside the body [10]. Thus, with these improvements the nanomedicines can be developed as the most widely and cost effective treatment for various deadly diseases.
Conclusions
The use of nanotechnology has proved to be a boon in the medical industry. It has led to the increased efficacy of the drug. The nanomedicines are far better than the conventional medicines because of their improved therapeutic and pharmacologic properties. Nanotechnological applications in drug delivery, diagnostic and treatment of diseases have made it convenient and possible to find treatment for diseases with high mortality rates. Nanoparticles led to the targeted delivery of drugs in a controlled manner only at the site where it is required, therefore, the effect of drug on other cells can be prevented and toxicity can be reduced significantly. It can also allow the drugs to pass through the cell barriers of highly specialized organs such as that of brain. The many advantages associated with the use of nanotechnology have made it a largely explored area that can provide us with better implementation of nanotechnology for the treatment of presently "incurable” diseases such as cancer and AIDS.
References
Copyright© Mahendra Kumar, Umesh Kumar, and Alak Kumar Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.