Research Article
Highly Sensitive and Selective Chromium Oxide Nanoparticles Modified Carbon Paste Membrane Electrode to Determine Cr Concentration in Different Solutions
Emad Salaam Abood 1, Ahmed Salim Abed 1, Zahraa Salman 1
1 Department of Medical Physics, Hilla University College, Babylon, Iraq.
* Corresponding author. E-mail: dr.emadsalaama@hilla-unc.edu.iq
Received: Sep. 7, 2021; Accepted: May 8, 2022; Published: May 8, 2022
Citation: Emad Salaam Abood, Ahmed Salim Abed, and Zahraa Salman, Highly Sensitive and Selective Chromium Oxide Nanoparticles Modified Carbon Paste Membrane Electrode to Determine Cr Concentration in Different Solutions. Nano Biomed. Eng., 2022, 14(1): 53-57.
DOI: 10.5101/nbe.v14i1.p53-57.
Abstract
Chromium ions in the solution were electro-oxidized using a novel carbon paste electrode containing Cr2O3 nanoparticles. As a result of the modification, the voltammetric response of Cr-ion could be resolved into one well-defined peak. As a result, the quest-reversible mechanism was activated. Researchers investigated the electrode's kinetics between 20 and 30 degrees Celsius. In the redox process of Cr-ion oxidation, the voltammogram data indicated a rise in temperature that increased negative shift, which implied diffusion electron transfer. The Randles-Sevcik equation gave a diffusion coefficient of 1.9 x10-3; the rate constant K was 0.3x10-3; and According to the study, In the range of 2-10 ppm, the peak current of Cr-ion rose linearly with its concentration, thermodynamic and kinetic parameters was calculated such as ∆E, ∆H, ∆G and ∆S equal to -58.2, -4.9, -65.1 and 0.11 KJ/mol respectively.
Keywords: CV, MONPsCPE, Cr2O3-NPs, Hydrothermal method, Chromium oxide nanoparticles
Introduction
A variety of uses are possible for transition metal oxide nanoparticles (TMONs), including catalysts [1-3], sensors [4-5], and superconductors [6]. It is a type of material used in environmental research, electrochemistry [7], and biology [8]. It is also used in chemical sensors and magnetism as well as other disciplines [9]. The relevance of chromium oxides in science and technology has recently garnered a lot of attention [10]. In addition, chromium may produce various oxides due to its multiple stable oxidation states (SOS). Chrome oxide (Cr2O3), which is crucial in certain applications such as high-temperature-resistance materials, has received special attention. The composition, structure, crystallinity, size, and shape of inorganic materials influence their inherent characteristics [11]. Synthesizing Cr2O3 nanoparticles has been done using several different ways such as chromium-oxidation-in-oxygen (OCO) and sonochemical method [12-13]. Chemical reduction in aqueous solvents [14], out of all the technologies described, has the most potential to be expanded to new applications due to its simplicity and low cost. NPs of Cr2O3 have been synthesized in the present study by hydrothermal method to prepare a highly sensitive and selective modified carbon electrode to determine a trace amount of chromium ion in various solutions. Cr2O3 nanoparticles may be synthesized using a variety of ways such as precipitating the metal chromium in oxygen, precipitation gelating the metal chromium [15-18]. In another hand, Cyclic Voltammetry (C.V) investigated Processes of reduction and oxidation of molecular species CV may also be used to investigate chemical processes. that are triggered by electron transfer, such as catalysis [19]. The electrochemical method of The current response of a redox-active solution to a linearly cycled potential sweep between two or more predefined values is measured using cyclic voltammetry [20]. So that in this present used cyclic voltammograms to study the mechanisms of electrons transition during oxidation-reduction prosses when applied a constant potential over the chromium oxide nanoparticles carbon paste electrode in chromium ion solutions. However, excessive chromium concentrations and long-term exposure can cause many cytotoxic and genotoxic consequences that impair the body's immune system. However, The mechanism of Cr(VI)-induced cytotoxicity is poorly understood. Several in vitro and in vivo studies have demonstrated that Cr (VI) causes stress caused by oxidation by increasing the formation of (ROS), as a result of which genomic DNA is damaged and lipids and proteins are oxidatively degraded. A cascade of biological processes occurs after Cr(VI)-induced oxidative stress, including increased superoxide anion and hydroxyl radical generation, enhanced lipid peroxidation and genomic DNA breakage, changed gene expression, modification of intracellular oxidized states, activation of protein kinase C, apoptotic cell death. [22]
Experimental
Preparation of chromium oxide nanoparticles by Hydro-thermal method
The huge surface area of chromium oxide NPs indicates enhanced reactivity [20]. chromium Sulfide tetrahydrate powder was dissolved in 10 mL of distilled water and added with 35 mL of iso-butanol. The aqueous-organic mixture was heated to 75°C before adding 0.5 M NaOH dropwise for 4 hours with steady stirring. Similarly, by applying the same methods followed in the first step. All of the chromium oxide nanoparticles that had been manufactured were rinsed with distilled water at 75°C and dried in a 150°C oven. The dry Cr2O3 nanoparticle precursors were calcined for 4 hours at 400°C [21]. Fig1 Shaw the TEM (INSPECT S50, Tehran University, Iran) The morphology of the produced molecules was determined using this method Cr2O3-NPs sample.
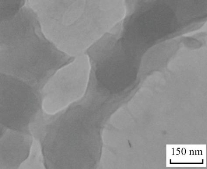
Fig.1 TEM for Cr2O3-NPs.
Chemicals and experimental apparatus
The electrochemical tests were performed carried out using a (Digi- ivy 2113 Potentiostat Texas, USA), and with system software. The chrome oxide NPs CPE was utilized as the chrome oxide (Merck Eng. 98%), and electrodes were made by combining (1.7 g, 3g and 1ml ) from ( Cr2O3-NPs, 3 g graphite, 1 mL paraffin oil) respectively, until an uniform paste was made in a mortar and pestle. A glass tube was used to hold the paste, which was inserted at the very end (diameter 0.25 mm length and 12 cm long). A gold wire was inserted into the carbon paste to create the electrical contact. A fine piece of paper was used to smooth the surface of the carbon paste before the experiment. However, no Cr2O3-NPs were added to the paste to generate the unaltered carbon paste membrane Fig.2 shows the diagram for electrode and cyclic voltammetry cell.
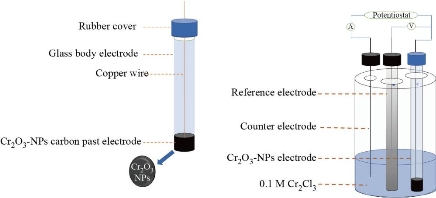
Fig. 2 Cr2O3-NPs carbon paste electrode insert with cyclic voltammetry cell.
Results and Discussion
With a chromium oxide nanoparticle membrane electrode in distal water, cyclic voltammetry (CV) was used to determine the electrochemical behavior of Cr2Cl3. For example, scan rate of 0.1 v. s-1, two distinct oxidation peaks were seen for Cr and Cr+2. This corresponds to anodic peak currents of around 0.14% and 0.18x10-5 volts for Epox1 and Epox2, respectively, which were measured. As a result of the anodic peak current Ipred=-0.102x10-5A, Epred=-0.471V was found to provide connectivity and a single reduction peak. This indicates that Cr2O3 NPs CPE was ISE for Cr ion.
Temperature Study
CV has examined the electro-oxidation of electrodes in the TEMP. limited of (20 - 30) °C. Cycle voltammetry a quasi-reversibility based on this, the kinetic and thermodynamic parameters were determined [20]. This is seen in Fig. 3 and Table 1, This demonstrates the obvious indication with the beaks changing to the negative side as scan rate increased, calculating Ea from slop lnD with 1/T K and the results which refer to physical endothelial reactions. ![]() an equation was used to compute the rate constant reaction. [21].
an equation was used to compute the rate constant reaction. [21].
At 20-30 °C, using the Nernst equation, the working electrode tacked the clear signal. (Eq. (1)).
![]() (1)
(1)
The free energy was estimated using the angle formed by ln D and 1/T K displayed in Fig. 4 and was found to be Ea = -58.2 KJ/mol. Finding demonstrated refer to Exothermic process.
By Equation (2), this result indicated the exothermic process that occurred when the working electrode was subjected to a voltage.
![]() (2)
(2)
The free Gibbs energy, which corresponded to the spontaneous reaction, was found to be -65.1 KJ/mol. Using Nicholson equation Eq., the reaction constant Kp was determined from the previous result (3)
![]() (3)
(3)
The first-order reaction of cyclic voltammetry, which is quasi-reversible, moves at 0.310-3 V/s. The physical characteristics to Cr2O3 NPs CPE are shown in Table 1. The outcome of the electrode surface. as shown in the Table 2.
Table 1 Voltammograms of I-E data for Cr2O3 NPs electrode Carbone passes electrode at 15, 20, 25, and 30 degrees Celsius, and diffusion coefficient for each step
|
Ln D |
D. cof. Cm2/s |
∆E = Epa2 - Epc/2 |
∆E = Epa - Epc |
Ipoxd A (10-5) |
Epc |
Ipoxd2 A (10-5) |
Epa2 |
Ipoxd1 A (10-5) |
Epa |
1/T 10-3 |
TEMP. K |
|
-20.9 |
21 - 10-6 |
- 0.15 |
- 0.30 |
0.8 |
1.1 |
0.84 |
-0.31 |
0.51 |
0.23 |
3.41 |
293 |
|
-21.5 |
15 - 10-6 |
-0.12 |
-0.24 |
0.5 |
0.9 |
0.59 |
-0.26 |
0.42 |
0.29 |
3.33 |
298 |
|
-21.4 |
14- 10-6 |
- 0.9 |
- 0.18 |
1.1 |
0.2 |
0.53 |
-0.38 |
0.51 |
0.26 |
3.30 |
303 |
Table 2 Kinetic variables of Cr2O3 NPs CPE
|
Type of electrode |
∆E KJ/mol |
∆H KJ/mol |
∆G KJ/mol |
∆S KJ/mol |
K S‑1 10-5 |
Do x 10-7 |
Α |
K0 |
|
Cr2O3 NPs Carbone past electrode |
-58.2 |
-4.9 |
-65.1 |
0.11 |
3.70 |
0.71 |
0.1 |
0.0003
|
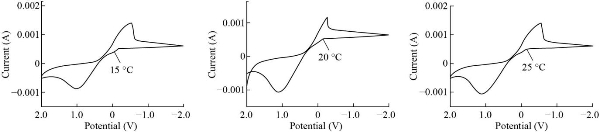
Fig. 3 Temperature influence on cyclic voltammograms on Cr2O3 NPs electrode Carbone past electrode with various: 20 degrees Celsius, 25 degrees Celsius, and 30 degrees Celsius, respectively.
Calibration curve
The calibration ranges were utilized to determine the concentration of chromium ion using CV. Chromium ion reaction to Cr2O3 nanoparticle electrode Cyclic voltammetry was used to study the carbon past electrode using various concentrations of pure stock solution, Fig.4 shows the calibration curve by CV in (1-5) parts per million, along with the equation w.t = (ppm/Mw/Mw of real drug sample)/(10 V), in ppm stands for range, and M.wt stands for molecular weight, along with the V which stands for volume, and the 10 represents the volume of the drug sample. To determine tiny amounts of medication components, the Cr2O3 NPs electrode Carbone past electro was shown to be more sensitive than colorimetric and atomic analyzers in terms of speed, economy, clarity, and safety. Chromium-containing vitamins (Hasanl A-Z Vital, Germany) stock solution and real sample results are shown in Table 3.
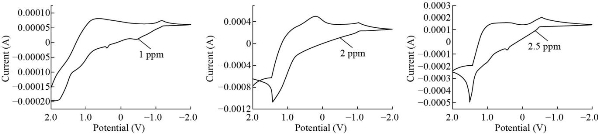
Fig. 4 Calibration curve by Cr2O3 NPs electrode Carbone past electrode for Cr2Cl3 solutions.
Table 3 The result of a deferent approach on a genuine drug sample
|
RSD % |
Founded |
Linear range |
Analyte |
Method |
|
|
|
10-3 – 10+3 ppm |
|
Cyclic voltammetry |
|
62.0% |
0.62 ppm |
|
Cr 1 ppm |
|
|
64.5% |
1.29 ppm |
|
Cr 2 ppm |
|
|
78.8% |
1.97 ppm |
|
Cr 2.5ppm |
|
|
|
|
0.08 – 10+3 ppm |
|
Coulometry |
|
51.0% |
0.51 ppm |
|
Cr 1 ppm |
|
|
55.0% |
1.10 ppm |
|
Cr 2 ppm |
|
|
75.6% |
1.89 ppm |
|
Cr 2.5ppm |
|
|
|
|
0.1 -10+3 ppm |
|
Atomic radiation |
|
83.0% |
0.83 ppm |
|
Cr 1 ppm |
|
|
96.0% |
1.92 ppm |
|
Cr 2 ppm |
|
|
95.6% |
2.39 ppm |
|
Cr 2.5ppm |
|
Conclusions
When put over the electrodes, these nanoparticles are extremely discerning and sensitive, detecting minute alterations in the current that passes through their blood. When you're used to create the electrode and examine its physico properties characteristics, as well as when employed to measure minuscule quantities of pharmaceuticals containing Cr, the findings of such usage were very accurate in comparison to standard methods as an example, consider the atomic analyzer and the colorimetric approach.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Emad Salaam Abood, Ahmed Salim Abed, and Zahraa Salman,. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.