Nanoparticles as Carriers in Medical Applications: A Review Focusing on the Preparation and Use of Nanoparticles in Tissue Regeneration
Andres Arias-Arana 1, Luis Palomino-Marcelo 1, 2, Miguel Gakiya-Teruya 1, Pamela Areche-Vargas 1, Anand Ramamurthi 3, Juan Carlos Rodriguez-Reyes 1, 2 *
1 Nanoscience and applications laboratory – NASCA, Department of Chemical Engineering, Universidad de Ingenieria y Tecnologia – UTEC, Jr. Medrano Silva 165, Barranco, Lima 04, Peru.
2 Bioengineering Research Center, Universidad de Ingenieria y Tecnologia – UTEC, Jr.Medrano Silva 165, Barranco, Lima 04, Peru.
3 Department of Bioengineering, Lehigh University, Bethlehem, Pennsylvania 18015, USA.
* Corresponding author. E-mail: jcrodriguez@utec.edu.pe
Received: Sep. 5, 2021; Accepted: May 8, 2022; Published: May 12, 2022
Citation: Andres Arias-Arana, Luis Palomino-Marcelo, Miguel Gakiya-Teruya, Pamela Areche-Vargas, Anand Ramamurthi, and Juan Carlos Rodriguez-Reyes, Nanoparticles as Carriers in Medical Applications: A Review Focusing on the Preparation and Use of Nanoparticles in Tissue Regeneration. Nano Biomed. Eng., 2022, 14(1): 90-106.
DOI: 10.5101/nbe.v14i1.p90-106.
Abstract
Nanotechnology and its applications to medicine, known as nanomedicine, involve a wide use of nanomaterials to stimulate and guide the regenerative properties of cells. In particular, tissue regeneration can be promoted by enabling a controlled release of therapeutic agents, which can be done using nanoparticles. In this review article, the fundamentals of tissue regeneration are discussed, focusing on epithelial tissue, to demonstrate the importance of delivering therapeutic agents in an efficient, sustained and localized manner. Then, the methods for synthesizing metallic and polymeric nanoparticles are described. While polymeric nanoparticles can be loaded with an agent during synthesis, metallic nanoparticles must first be synthesized to later interact with therapeutic agents. This interaction can be fine-tuned by functionalizing metallic nanoparticles with organic molecules, which results in a more controlled attachment.This review highlights the importance of choosing the appropriate method of synthesis and functionalization, which must be designed considering both the type of tissue to regenerate and the nature of the agent to be transported.
Keywords: Nanomedicine, Nanoparticles, Tissue regeneration, Peptides
Introduction: Nanomaterials and Their Uses in Tissue Regeneration
Nanotechnology is defined as the creation and use of materials, devices and systems through the manipulation of matter at the nanoscale, i.e., in the size range between 1 and 100 nm [1]. Its goal is to understand, create and exploit the new properties and functions acquired by matter below the microscopic scale [1]. As an example of the difference in properties due to size, gold nanoparticles (AuNPs) have been observed to act as catalytic agents even though gold is inert in its bulk phase [2]. In a biological context, nanoparticles (NPs) provoke additional interest due to their ability to cross cellular barriers, which is an advantage for drug delivery [3]. The use of nanomaterials in the biomedical sciences towards medical solutions is known as nanomedicine [2], and has driven advances in medical diagnosis, nanodrugs and tissue regeneration. In medical diagnosis, nanomaterials are used as part of biosensors for in vitro sensing applications due to the high sensitivity exhibited by metals such as gold and silver, originated from surface plasmon resonance effects [4]. Also, nanoparticles can be employed as contrast agents of low toxicity and high sensitivity for in vivo imaging-based diagnosis and monitoring of tissue process [5]. In particular, magnetic nanoparticles (e.g., magnetic iron oxide nanoparticles, MION) can be used for labeling and tracking cell growth and delivery [6]. On the other hand, nanodrugs are nanomaterials serving as carriers of drugs or biological agents to specific parts of the body with the aim of stimulating, suppressing, or modulating certain biological processes [7]. For example, NPs can transport an anticancer drug or a radionuclide to cancer cells, interacting with the cell’s receptors through specific ligands such as peptides, proteins, antigens, etc. Tissue regeneration refers to regenerative therapies to promote the growth of tissue through the use of scaffolds and the delivery of therapeutic agents [8]. Both strategies are currently heavily dependent on nanomaterials. Scaffolds are structures where cell growth is promoted and, therefore, one of the challenges associated with its use is to design materials with the desired mechanical, chemical and biological properties. Scaffolds can be made out of metals (such as titanium) or polymers (hydrogels), with the latter option being preferred as their properties can be better tuned during synthesis and they can be made from biodegradable materials [9,10]. Hydrogels can be synthesized as macroscopic materials or as nanoscopic materials (nanofilms or nanofibers), by using starting materials such as polyvinyl alcohol (PVA), polyethylene glycol (PEG) and polylactic acid (PLA). Nanofibers can mimic the extracellular matrix, environment in which cells grow and thrive. Further improvement in a macro-scale scaffold can be achieved by including nanoparticles in the macrostructure and/or therapeutic agents which can be released controllably. It has been demonstrated that metal and metal oxide nanoparticles can favor several processes of interest for tissue regeneration [9,11]. On the other hand, nanomaterials can also work as carriers of therapeutic agents and biological material such as proteins, DNA and genes in order to regenerate an affected area [12,13]. In the case of tissue regeneration, the appropriate delivery of biomolecules such as cytokines or growth factors is crucial for the healing process, and so it is the supply of key building blocks for the regenerated tissue. Due to their ability to serve as component of scaffolds and as drug carrier, nanomaterials have been instrumental in the development of the multidisciplinary, novel field of tissue engineering and regenerative medicine (TERM), which aims to improving the regeneration process and to restore normal biological functions in damaged tissues [8,9]. Recently, there have been high-quality literature reviews focused on summarizing the results of the application of nano-based technologies in tissue regeneration. These articles are often directed towards the use of nanomaterials to direct cell growth in scaffolds (e.g. for vascular tissue [14], skin rejuvenation [9], bone regeneration [10,11,15,16] and periodontal tissue [17,18], etc.). However, these reviews do not focus on the synthesis and functionalization of nanoparticles for drug delivery in efficient, localized, and sustained manners. The present review intends to fill this void and will serve as complement to other reviews more focused on specific applications.
Concepts and Mechanisms in Epithelial Tissue Regeneration
General concepts in tissue regeneration
Epithelial tissue regeneration is an example of a vital physiological process that must be therapeutically stimulated to ensure a rapid and safe healing process. This generally includes four phases: hemostasis, inflammation, proliferation and remodeling. These processes are schematically shown in Fig. 1 [19], indicating various biomolecules and agents involved in each stage (the most relevant to our discussion are described in more detail in Section 2.2). (A) Hemostasis, is the process by which bleeding stops and takes place immediately after the injury and is relatively short-lived (4 to 6 hours). Its exact duration depends on the nature of the injury and the type of tissue affected. Blood vessels undergo vasoconstriction and platelets adhere (with the help of collagen) to the damaged vessel, and later clotting factors complete the formation of the clot. (B) Inflammation, where a series of inflammatory events, essential for settling the bases of regeneration, are chemically mediated by the temporal-spatial expression pattern of specific cytokines and growth factors (GFs) [20]. In particular, inflammatory cells, known as fibroblasts, initiate the segregation of collagen, elastin, hyaluronic acid and other proteins to start regeneration [14,21]. Fibroblasts also release several factors for promoting the formation of blood vessels (proangiogenic factors). (C) Proliferation, involves the generation of repair material, where stem cells migrate to the affected area, proliferate and differentiate. For most musculoskeletal injuries, scar tissue starts to be formed from stem cells and fibroblasts. It has a rapid onset time (24-48 hours) but reaches peak activity usually between 2-3 weeks after the injury. At this point, blood vessels and capillaries are also rebuilt (angiogenesis), which ensures the appropriate delivery of oxygen and nutrients to the area under reconstruction. (D) Remodeling, where the extracellular matrix is restored, lasting until the tissue has morphed into an organized, high-quality and functional scar that is capable of behaving similarly to the parent tissue [19]. It should be noted that all cutaneous wounds that extend deep into the dermis and are larger than 1 cm in diameter require specialized treatment. In the case that the wound is unable to close by itself, extensive scars can result in both mobility limitations and cosmetic deformities [22]. Due to the importance of bioactive peptides in tissue regeneration as shown in Fig. 1, their transport has become important in TERM; currently there are more than 100 commercially available peptide- based therapeutics approved for clinical use. However, their clinical efficacy is limited by challenges to readily absorb the peptides into tissues: a rapid degradation of the peptide, and a poor specificity of delivery [23]. For this reason, the objective of peptide delivery from a carrier is to avoid degradation and non-selective accumulation of the protein, maintaining its solubility and biological activity, and achieving a controlled distribution on the site of action [24]. To achieve this, NPs are widely investigated as drug delivery vehicles due to their small size and large surface area/volume ratio to enable rapid release, ability to be actively targeted, and small degradation footprint (in case of biodegradable polymer NPs).
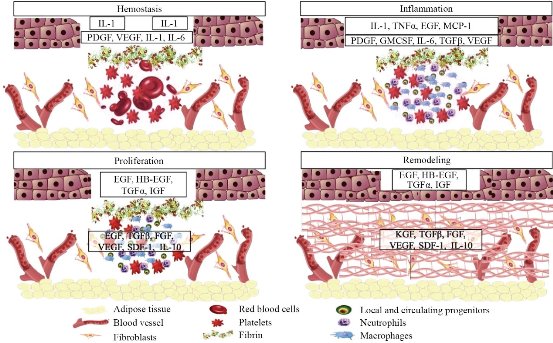
Fig. 1. Phases in the healing of skin wounds: hemostasis, inflammation, proliferation and remodeling. Key cell types and proteins involved in this healing are listed at the bottom of the figure. Also indicated are key cytokine modulators of tissue healing, which include IL-1 (interleukin-1), IL-6 (interleukin-6), IL-10 (interleukin-10), PDGF (platelet-derived growth factor), VEGF (vascular endothelial growth factor), TNFα (tumor necrosis factor alpha), EGF (epidermal growth factor), MCP- 1 (monocyte chemoattractant protein-1), GMCSF (granulocyte-macrophage colony stimulating factor), TGFβ (transforming growth factor beta), HB-EGF (heparin-binding EGF-like growth factor), TGFα (transforming growth factor alpha), FGF (fibroblast growth factors), SDF-1 (stromal cell-derived factor-1), IGF (insulin-like growth factor) and KGF (keratinocyte growth factor). Adapted from “Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications”, by S. Hamdan, I. Pastar, S. Drakulich, E. Dikici, M. Tomic-Canic, S. Deo, and S. Daunert, 2017, ACS Central Science.
Nanoparticles possess certain traits that make them attractive alternatives as carriers of therapeutic agents during tissue regeneration. Some of these include their small size and their high surface to volume ratio which provide a high probability of interaction with biological substrates, as well as high drug release rates. In addition, the small size of NPs enables their enhanced penetration into a variety of tissues and their intracellular uptake. That implies a decrease of doses during a treatment. Other advantages of NPs include the protection of the therapeutic agent towards maintaining its stability in vivo, and also the protection against immune responses triggered by chronic exposure to unencapsulated drugs [7]. Nanoparticles can exhibit a variety of sizes, architectural designs, stabilities, drug release rates, control mechanisms, shapes and other characteristics. These properties must be fine-tuned to respond to a specific set of requirements from the therapeutic agent and from the biological environment to which they will be targeted for therapeutic action. Regardless of their characteristics, NPs must present three main features:
(A) The interactions between the NPs and the drug or biological material must be strong enough to be transported to the desired area, but also weak enough to be released once it reaches the area of interest.
(B) The NPs must be of an adequate size which is mainly related to the specific area where the drug is to be delivered. For example, if one desires to deliver the drug into the brain, the transport vector must be smaller than 10 nm [40].
(C) The NPs must be able to be directed towards the desired area. This control can be passive (for example, through diffusion) or active (for example, applying a magnetic field to magnetic NPs) [6, 40].
Nanoparticles also present advantages regarding safety. Several NPs types, such as biodegradable NPs, present limited cytotoxicity in human systems due to their small biodegradation footprint , and show little accumulation in the body [7]. Also, some nanoparticles can be directed to specific sites by external forces, such as magnetic fields for magnetic nanoparticles, which allows for a controlled delivery to a designated area [6]. However, it is important to mention that some nanoparticles raise concern due to their cytotoxicity. For example, AgNPs can disrupt cellular processes, either by the attachment of the nanoparticle to the membrane or to its internal organelles, or by forming and releasing metal ions and/or reactive oxygen species [41]. Other nanoparticles, such as AuNPs, are often assumed to be inert and biocompatible, and others such as iron oxide are assumed to be innocuous as they generate iron ions with limited side effects. Nonetheless, it is always important to confirm that nanomaterials and their decomposition products do not interfere with other biological processes or bioaccumulate in surrounding organs. An additional advantage is that the NP’s surface can be modified with biomolecular ligands which allows them to be actively targeted to specific areas in biological systems, giving specificity and selectivity during drug delivery [7,8,39,42]. They can be modified with biomolecules such as peptides, proteins, ribonucleic acids and polymers which increases their biocompatibility and reduces their toxicity. In particular, peptides are the ideal attachable biomolecules because they can chemically ‘recognize’ a cell through specific ligand-receptor interactions on cell membranes. Polymeric NPs can be loaded with a myriad of therapeutic agents [43]. The polymers can protect the biomolecules from undesired reactions in the surroundings, improving their stability, reducing the activation of the immune system, and extending the circulation time within the organism [44]. Thus, proteins and peptides administered through the use of polymeric nanoparticles (PNPs) reduce the frequency of doses and conduct to an improved regeneration. In particular, the use of peptides-loaded poly (lactic-co-glycolic acid) nanoparticles (PLGA NPs) has shown highlighted results. For instance, the application of systems based in VEGF and FGF-loaded PLGA NPs improved the number and arrangement of regenerated urothelial cells and smooth muscle cells as well as microvascular density and maturity during bladder tissue regeneration in a rabbit model [45]. More recently, IGF-loaded PLGA NPs incorporated to 3D-printed poly-ε-caprolactone scaffold showed a higher cell proliferation and better compatibility in cartilage tissue engineering [46]. Regarding metallic NPs, those made of gold and silver are the most promising and explored tools in nanomedicine. In fact, AuNPs in TERM can be used as multimodal tools to improve the properties of scaffolds helping in cellular differentiation and in the delivery of GFs [47]. In the first reported example of intracellular NPs delivery, modified albumin was attached to AuNPs and later delivered to the cytoplasm and nuclei of functional cells [48]. On the other hand, AgNPs have been used in topical application or impregnated in scaffolds where their antimicrobial and anti-inflammatory properties accelerate the repair of wounds [49]. The most commonly used metallic NPs available commercially are AgNPs [50,51]. These NPs have broad antibacterial activity against various bacteria including those that are multi-resistant to antibiotics [52,53]. AuNPs have been widely studied as transport vectors, but in recent years it has been shown that they also display bactericidal properties against enterobacteria [54] and Gram-positive bacteria [55]. Furthermore, it has also been shown that bimetallic Ag@Au NPs (a silver core within a gold shell) have greater antibacterial power than AgNPs and AuNPs alone [56], which opens the possibility of tuning the antibacterial properties by controlling the ratio Au/Ag. The bactericidal power of both AgNPs and AuNPs depends on their shape, size [55] and the dose supplied. It has been suggested that the antibacterial power increases as their size decreases [50,54], due to the fact that smaller NPs have a greater surface energy [50], which means that they have greater reactivity [57] and, therefore, react at a faster rate with bacteria. There are four possible mechanisms by which AgNPs can act on bacteria [50,58]:
(A) The accumulation and dissolution of NPs in the bacterial membrane can change their permeability with a subsequent release of intracellular biomolecules [58].
(B) The generation of reactive oxygen species (ROS) inside the cell causes subsequent oxidative damage [58].
(C) The intake of metallic ions and NPs into the cell results in disruption of ATP production and DNA damage [58].
(D) The NPs and their ions can bind to and disrupt enzymes inside the cell leading to an arrest in cellular respiration [58].
As it was previously mentioned, in TERM there is an intrinsic need to maintain the area of tissue repair with the least possible exposure to microorganisms and to avoid the formation of bacterial biofilms. The elimination of these biofilms has always been a challenge due to their resistant nature to conventional antimicrobial therapies and also to the need for not interfering with the healing process [26,59]. It is in this context that antimicrobial NPs are useful with prospects to function as both carrier agents and bactericidal materials.
Overview of metallic and polymeric nanoparticle synthesis
In general, strategies for NP synthesis can be defined according to the starting compound or according to the synthesis method which can be either top-down or bottom-up. In the top-down approach, macro or microscopic blocks are used as starting material which are then fragmented until nanoscale particles are obtained. Conversely, in the bottom-up approach, the process begins with molecules or ions which nucleate and grow to form NPs. These strategies are further classified as physical, biological or chemical methods.
Physical methods.
In these methods, materials are exposed to mechanical, electromagnetic, thermic or electric energy to generate NPs by abrasion, ablation, photolysis or evaporation/condensation. Most of them follow a top-down approach, such as a laser ablation, procedure wherein a solid is exposed to a laser powerful enough to heavily degrade the solid´s structure. This results in the release of NPs into a stabilizing medium or onto a target substrate. Other top-down procedures include: a) ultra-fine grinding, where a precursor compound is mechanically activated to form small clusters which grow in size to become NPs [60]; b) photolysis, where a substrate can form NPs when it is exposed to visible light [61]; and, c) evaporation/condensation, where a substrate is irradiated with energy from thermal or electric sources, emitting gaseous atoms which are condensed on the desired surface [62]. In general, these methods do not require a solvent and produce NPs whose size depends on controllable parameters such as voltage, intensity/frequency of the electromagnetic waves, time and temperature. Nonetheless, they often result in an elevated cost due to the highly specialized energy sources required.
Biological methods
These methods are based on the generation of by-products as a result of the metabolism of living organisms (bacteria, fungi, etc.) by being exposed to media containing NPs precursors [63]. Depending on the precursors, these strategies can be considered top-down or bottom-up. While in biological top-down methods the microorganisms produce NPs from ingested portions of a substrate, bottom-up assembly involves ingestion of ionic precursors which are then metabolized and ejected as NPs. Both methods are considered advantageous due to their low toxicity, cost effectiveness and efficiency [64].
Chemical methods
Despite the existence of a wide variety of chemical reactions that lead to the formation of NPs, the most used are based on the reduction of metal ions by combining them with reducing agents in a solution. An increased control over the properties of the resulting NPs is achieved using selected compounds such as: chloroauric acid (HAuCl4) and sodium citrate in the Turkevich method or silver nitrate (AgNO3) and sodium borohydride in the Creighton method [65,66]. Reduction can be facilitated photochemically, electrochemically or by applying sonication [61]. The success of these methods depends not only on the precursor agent, but also on the choice of solvent, reducing substance, presence/absence of stabilizing agents and other physical-chemical parameters as concentration, temperature, etc. [67–70]. Table 1 shows the impact that the precursor agent and solvent have on the morphology and size of AgNPs. More advanced methods for the synthesis of NPs by chemical means include the use of vapor phase techniques, such as chemical vapor deposition or atomic layer deposition, where two precursor substances are deposited on a substrate, typically, at high temperatures and involve reactions that produce NPs and gaseous byproducts [62]. These techniques are restricted to the design and development of catalysts due to their high cost.
Table 1. Chemical reduction methods to synthesize AgNPs and the morphology and size of resulting particles.
|
Solvent /Reducing agent |
Stabilizing agent |
Morphology and particle size |
References |
|
H2O /Sodium citrate |
Sodium Citrate |
Nanofibers (30 nm diameter) |
[71] |
|
N,N- dimethylformamide |
Polyvinylpyridone |
Nanoprisms & nanospheres |
[71] |
|
H2O/NaBH4 |
Dodecanethiol |
Nanospheres |
[71] |
|
H2O /Tollens reactant |
- |
Nanospheres (20-50 nm) |
[71] |
|
Acetonitrile |
Polyvinylpyridine |
Dendritic particles |
[71] |
|
H2O /Sodium citrate |
Sodium citrate |
Nanospheres (30-50 nm) |
[72] |
Polymeric nanoparticles (PNPs) and microparticles (PMPs) are classified according to their size and function. The PMPs vary in diameter from 1 to 250 μm, while the size of PNPs ranges between 10 and 1000 nm [73]. Emulsion solvent evaporation techniques are frequently used to produce PNPs and PMPs which typically requires the following compounds:
Polymer: Must be dispersed in a solvent. Polymeric properties and molecular mass determine the final shape and size of the NPs. The ones used most frequently include poly (lactic-co- glycolic acid) (PLGA), polyvinyl alcohol (PVA), alginates, polyethylene glycol (PEG), etc. [25,74].
Stabilizer: Compound that interacts with the growing NPs to determine its size, usually limiting it. Stabilizers can help by forming dendritic or micellar arrangements [75–77].
Solvent: Medium in which polymers are dispersed for subsequent nucleation. Apart from the aqueous medium, polymers can be suspended in alcohols or apolar solvents such as chloroform, benzene, etc. [75, 78].
There are three methods mainly used to encapsulate a biomolecule within PLGA NPs/MPs: water-oil- water emulsion technique (w/o/w), phase separation methods and spray drying [79]. The first method is presented schematically in Fig. 3, starting with peptides or proteins in an aqueous solution which are dispersed in an organic solution of PLGA, producing a water-in-oil emulsion (w/o). This first dispersion stage is carried out using high speed homogenizers or sonication. MPs and NPs are produced either by extracting the organic solvent once this first w/o emulsion has been dispersed in a stabilizing aqueous solution or by adding a non-solvent such as silicone oil, thus inducing coacervation. This first process is often referred to as the water/oil/water (w/o/w) method, while the latter is known as the phase separation technique. In both cases, particle formation occurs in the liquid phase. In the spray drying technique, particle formation is achieved by atomizing the emulsion in a stream of hot air under a strong evaporation of the solvent [80]. An important aspect to consider is that most proteins and peptides are hydrophilic and any attempt to load them into PLGA (hydrophobic) becomes a difficult task. To solve this problem, the use of a medium is required. Although the o/w method is widely used, frequently the peptides are expelled from the hydrophobic PLGA into the water dispersion medium during mixing, resulting in hardened particles [81]. To minimize such partition of the drug between two immiscible phases, the double emulsion method w/o/w is the most used for the synthesis of PLGA NPs loaded with bioactive peptides. However, peptides encapsulated by w/o/w in PLGA NPs and MPs are susceptible to denaturation, aggregation, oxidation and cleavage. These problems have been overcome by the addition of stabilizers such as carrier proteins, for example albumin, or PVA during the primary emulsion phase, or halogen and manganese sulfate molecules in the protein phase [80]. Fig. 4 shows the general scheme of PLGA NPs synthesis, following the methodology described in the literature [28,75,78]. This consists of the suspension of the PLGA polymer in chloroform, followed by the addition of the selected peptide which may be in aqueous solution. Using an ultrasound probe, a water-in-oil emulsion can be produced where the peptide will be in the aqueous phase and the PLGA polymer encapsulates it. This emulsion is subsequently added to an aqueous solution of polyvinyl alcohol and ultrasound is applied again, so that a water-in-oil-in-water emulsion is obtained. The sonication is carried out at 0 ° C to avoid any temperature increase due to the effect of ultrasound for two minutes, after which the emulsion is stirred for 16 hours at room temperature and is finally dried to remove organic solvents. After an ultracentrifugation, the NPs are separated from the solvent.
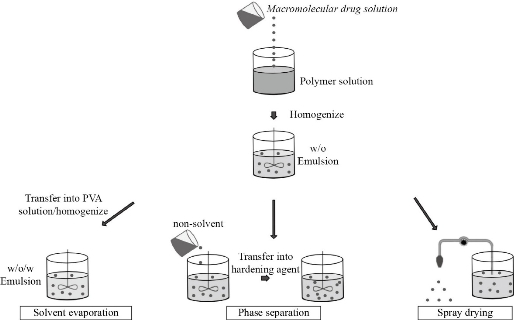
Fig. 3 Nano/microencapsulation methods: (left) water / oil / water emulsion, (center) polymer phase separation and (right) spray drying. The aqueous solution is dispersed in the polymeric solution through a w/o ultrasound emulsion. Adapted from “Nano/micro technologies for delivering macromolecular therapeutics using poly (D, L-lactide- co -glycolide) and its derivatives”, by R. C. Mundargi, V. R. Babu, V. Rangaswamy, P. Patel, and T. M. Aminabhavi, 2008, Journal of Controlled Release.
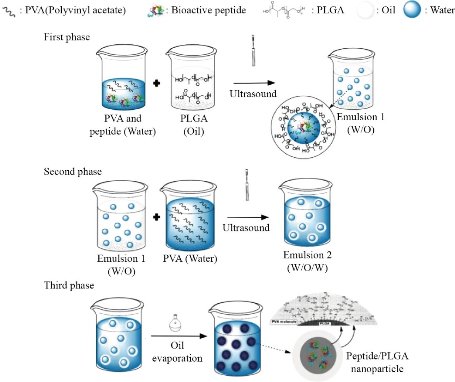
Fig. 4 Schematics of PLGA NP synthesis. The first step represents the Emulsion 1 (water-in-oil) formation, which is used in the second step to prepare Emulsion 2 (water-in-oil-in-water). The third step represents oil evaporation to achieve the encapsulation of the peptide/drug.
(A) Solvents: Water is generally considered the best solvent available for NPs synthesis. However, it is also possible to use organic solvents to have better control over the size and shape of the resulting NPs [82]. The use of organic solvents, however, raise environmental concerns as their disposal is often difficult.
(B) Reducers: Synthesis by oxidation-reduction (redox) reactions generally uses sodium borohydride, hydrazine, ascorbic acid, cethyltrimelthylammonium bromide, polyvinyl alcohol and polyvinylpyridine as reducing agents and stabilizers. It is important to take into consideration that many of the potential reducers display some degree of toxicity in medical and environmental applications. Therefore, there is an active initiative to use the so-called “green reducers” [83,84] such as glucose, sucrose and some polysaccharides such as heparin, hyaluronic acid, starch, cellulose, alginic acid, dextran and chitosan [83]. The reducing capacity of carbohydrates and polysaccharides is associated with the presence of reducing groups such as aldehydes. In addition, alcohol groups (-OH) present in large quantities in polysaccharides of certain algae can also act as reducers [85].
(C) Stabilizers: Stabilizing agents are constituted by molecules capable of adhering to the growing suspended NPs or macromolecules capable of immobilizing the NPs in a matrix. In both cases, stabilizers reduce the reactivity of the growing NPs, limiting their growth and morphing them into specific shapes [68]. The molecules chosen as stabilizers must have some reactive group that allows for a strong interaction to the NPs. In the case of AuNPs, for example, most stabilizers have a thiol group (-SH) due to the strength of the gold-sulfur interaction [76,82,86]. Stabilizer polymers can also work by forming stable bonds through intermolecular interactions, such as van der Waals forces in the case of interaction between AuNPs and aromatic groups (- C6H5) [87]. The use of polymers avoid the formation of formal chemical bonds, which can modify the electronic properties of the NPs [61,62,68]. In some cases, the reducer can also work as a stabilizer. For example, in the case of sodium borohydride where the borohydride anion covers the NPs and endows them with a negative surface charge that causes a repelling effect among the NPs [68,69].
Among the great variety of purely chemical methods for NPs synthesis, this review will focus on reductions using sodium citrate and sodium borohydride.
This method is used for the preparation of metallic NPs from many transition and heavy metals such as Au, Ag, Cu, Pt, Rh, Pd, Fe, Co and Ni, among others. Its reduction efficiency increases with a decrease in pH as a result of the hydrolysis of BH4- which results in the formation of intermediate products with more pronounced reducing properties [88]. The combination of sodium borohydride with stabilizing agents can result in the production of small NPs that aggregate slowly. For example, the synthesis of monodispersed spherical AgNPs has been pursued with different ratios of borohydride to silver ion concentration ([BH4-]/[Ag+]), all of which were stable for at least 1 year [89]. The system was controlled by measuring the absorption spectra and initial observations revealed that the NPs had an average size of 5 nm. Their mean size did not undergo changes with a quadruple variation of the concentration ratio mentioned beforehand. Over time, there was an increase in the maximum optical density and a redshift of the absorption band, the latter indicates the increase in particle size. The reduction of silver ions by borohydride ions is shown in Equation 1: Reduction of silver ions by borohydride [63].
2𝐴𝑔+ + 2𝐵𝐻4− → 2𝐴𝑔 + 2(𝑂𝐻)3 + 𝐻2. (1)
The analysis of efficiency in NPs synthesis can be carried out using UV-VIS spectroscopy. Different NPs shapes and sizes generate characteristic absorptions in the visible range due to plasmonic effects on both elements in the nanoscale [66,67,70] . Fig. 5 shows a scheme emphasizing the steps involved in the mechanism of formation of AgNPs: reduction, nucleation and growth [68].
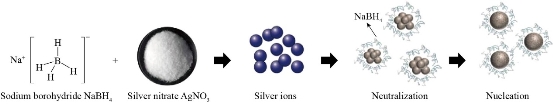
Fig. 5 Schematic representation of synthesis of AgNPs by redox using sodium borohydride as a reducing agent and silver nitrate as a precursor agent.
Sodium citrate acts as a reducing and stabilizing agent. Therefore, its concentration determines the rate of reduction, the growth kinetics and the NPs stability. This method is generally used for the preparation of AuNPs, although this can also be applied to the synthesis of AgNPs. The reaction of the metal ion reduction for Ag using sodium citrate as reducing agent is shown in Equation 2: Reduction of silver ion by sodium citrate [63]:
4𝐴𝑔+ + 𝐶6𝐻5𝑂7𝑁𝑎3 + 2𝐻2𝑂 → 4𝐴𝑔 + 𝐶6𝐻5𝑂7𝐻3 + 3𝑁𝑎+ + 𝐻+ + 𝑂2. (2)
The synthesis takes place at the boiling point of the aqueous solution of the reagent mixture which is 100 C at atmospheric pressure. The NPs formation process is strongly affected by the ratio of metal ions to the concentration of reducing agent, the pH of the solution, the boiling time and the mixing speed of the reagents [72]. If the citrate concentration is insufficient to stabilize the clumps present in solution, particle growth occurs through aggregation. Furthermore, the speed of reagent mixing affects the rate of cluster formation and the growth of NPs. For example, rapid injection of the reducing agent leads to extremely quick nucleus formation, followed by slower growth. Due to the high concentration of nuclei, the probability of their aggregation increases, and this results in a greater mean NPs size. In contrast, when citrate is gradually injected, the rate of reduction of metal ions decreases, the processes of nucleus formation and NPs growth proceed simultaneously and the probability of aggregation and NPs size are smaller than in the previous case [90]. The average size and degree of polydispersity of the NPs decrease as pH values increase in the range of 4.5-6.5, presumably due to the increase in the negative charge density of the NPs, which better stabilizes the citrate protection, to ultimately prevent aggregation. Additionally, the shape of the NPs also depends on the pH. For example, at pH 4.5, polyhedral and ellipsoids NPs are formed, at 5 <pH <6 predominantly ellipsoids NPs, and at pH 6.5, spheroids NPs [91]. The citrate synthesis method for AgNPs leads to a slower reduction and a tendency to aggregation; however, its use is preferred when one is looking for an alternative method of low toxicity. The variation in the concentration of citrate, silver salt and pH leads to a change in the shape of the NPs [71]. For example, the synthesis of AgNPs using citrate at pH 9 and using NaOH to regulate the pH results in the formation of nanobars or nanowires which have a lower antibacterial power than spherical NPs formed at pH 6 [73].
Nanoparticle functionalization involves the modification of the NP’s surface using various types of molecules which can attach themselves to such surface. Specific surface functionalization strategies are required for each possible application because these interactions affect both the stability of the NPs and their physicochemical properties [92]. Functionalized nanomaterials designed to perform specific reactions are of great interest in medical diagnostics, drug delivery and catalyst applications [93]. Although many NPs have excellent physicochemical properties, they may not have adequate surface properties for specific environments or conditions [92,94]. Moreover, the controlled functionalization of NP surfaces with a suitable molecule changes the structure of the material, as well as its surface composition and morphology, leaving the bulk properties of the NPs intact. It is also known that surface modification of nanomaterials is essential, as this layer facilitates the reduction of surface energy by providing a protective coating that prevents the NPs from agglomerating and growing uncontrollably [92]. One NP type whose functionalization has been studied extensively is the AuNPs, because their functionalization has a wide range of applications in biology, nanomedicine, optics [95] and catalysis [96]. The functionalization of AuNPs can be carried out by different techniques such as direct synthesis [97], ligand exchange reaction [98] and high temperature processes [99]. Most strategies for AuNP functionalization rely on the use of thiol-terminated (-SH) molecules, since the affinity between gold and sulfur spontaneously produces Au-S bonds. For example, the modification of small interfering RNA (siRNA) with thiol groups (SH-siRNA) to direct its conjugation with AuNPs for cellular delivery has been reported [100]. Furthermore, other inorganic entities such as fullerenes (C60), carborane clusters, and oligomeric silsesquioxanes (POSS) are typically modified with thiol groups to functionalize them onto AuNPs, achieving the repulsion between the AuNPs and modifying their surface [101].
Peptide-nanoparticle Interactions
Biomolecules can be coupled to NPs following direct or indirect methods. A direct method is characterized by the addition of biomolecules to a suspension of bare NPs. These can be adsorbed onto the surface of the NPs by electrostatic, hydrophobic, van der Waals and coordination interactions [102,103]. Electrostatic interactions occur between positively charged biomolecule residues with the surface of the negatively charged NPs. Hydrophobic interactions occur when the biomolecule is adsorbed onto the surface of the NPs through its hydrophobic moieties. On the other hand, Van der Waals interactions occur when biomolecules are adsorbed onto the NPs but through dipole attraction. Finally, the coordination interactions occur when a coordinate bond is established between the NPs and the biomolecules [102]. Direct methods for coupling biomolecules to NPs are relatively simple and ensure a convenient NPs-peptide interaction. In the case of indirect methods, a molecule connecting the NPs with a biomolecule, hereafter known as a linker, is employed to be bonded via coordination interaction with the NPs. Then, by means of a reaction, it will form a covalent bond with the biomolecule [102]. This will allow the union between the biomolecules and the NPs to be more stable against dissociation which is important for many applications [103]. Some NP-peptide conjugations through covalent bonding are shown in Fig. 6. When it is desired to load NPs with biomolecules keeping their biological activity, it is preferable to use indirect methods. Even though the direct methods are simpler to perform, their enormous disadvantage is their possibility of denaturing the structure of the biomolecule, compromising its biological activity. Nevertheless, it could depend on the biomolecule’s nature, molar ratio of biomolecules to NPs and the conditions of the medium in which they are interacting. For example, previous studies of direct interaction between bovine serum albumin (BSA), a standard protein employed due to its high affinity to inorganic NPs [104],[105], and AgNPs demonstrate that BSA mainly interacts with AgNPs through van der Waals forces and it keeps its native-like structure in defined molar ranges which depend on the pH of the medium [105],[106],[107]. It is likely that BSA can also improve the stability of AgNPs, similarly to the reported for other NPs, such as Fe3O4 [103,108]. It is worth mentioning that a NP can modify the structure of a biomolecule due to the various interactions that may exist, for example, between the functional groups in the molecule and the surface of the NP. To avoid structural modifications in the biomolecule, it is possible to elect specific groups able to react with the NPs in a controlled fashion. For example, proteins with a cysteine group can use this moiety to bind to the NPs favorably, reducing the possibilities of other undesired interactions [109]. Numerous efforts have been developed to prevent protein denaturation during its coupling. The selection of an appropriate linker is crucial so that the biomolecule does not undergo denaturation. One of the most commonly used linkers is oligo ethylene glycol (OEG) since it reduces non-specific interactions, as well as provides additional degrees of freedom for terminal functional groups [103]. Once the NPs are functionalized with the linker molecule, a protein can be attached to the functionalized NPs by several methods. One of these is the standard amine-carboxylate coupling method [110]. Another method that has been employed is carbodiimide coupling [43,111]. A more recent method that has been used is the “click” reaction which consists of the copper-catalyzed reaction of an azide and a alkyne [103]. This method has been used to bind NPs to biomolecules such as lipase [112,113], luciferase [114], peroxidase [115] and antibodies [116] without losing their biological activity. There are other types of click reactions such as uncatalyzed thiol-amine directional coupling [116]. Even though the copper-catalyzed click reaction is very popular, this catalyst can inhibit the protein’s activity and is toxic to the host in certain doses, which is problematic when the reaction is carried out in vivo [117]. In spite of this, the rapid development of click reactions is evident from its use in a variety of disciplines, including medicinal chemistry, materials and polymer science due to its great advantages, such as its high nanoparticle-to-biomolecule coupling performance [103,118].
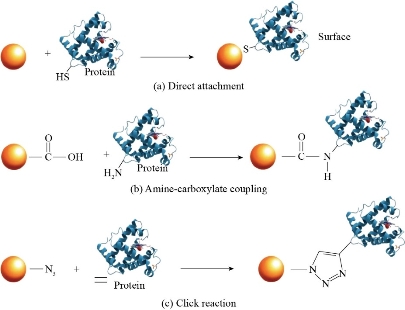
Fig. 6 NP-protein conjugation by forming a covalent bond. (a) Direct union of the protein’s thiol group onto the NP’s surface. (b) Amine-carboxylate coupling. (c) NP-protein coupling using the click reaction. Adapted from “Engineering the nanoparticle-protein interface: Applications and possibilities”, by R. Subinoy, Y. Yi Cheun, and V.M. Rotello, 2010, Current Opinion in Chemical Biology.
Acknowledgements
This work has been funded by FONDECYT – CONCYTEC (155-2015-FONDECYT) and by the partnership Cleveland Clinic - Universidad de Ingenieria y Tecnologia - UTEC. Karinna Visurraga and Luz Perez (UTEC) are acknowledged for administrative and technical support. Julio Valdivia, Javier Paino, Marco Málaga and Ana Luisa Alvarez (UTEC), and Vijay Krishna (Cleveland Clinic) are acknowledged for their support through the development of the project. Danae Chipoco and Layla Gonzales (UTEC) are especially thanked for their help in proofreading the manuscript.
Conflict of Interests
The authors declare that no competing interest exists.
References
Copyright© Andres Arias-Arana, Luis Palomino-Marcelo, Miguel Gakiya-Teruya, Pamela Areche-Vargas, Anand Ramamurthi, and Juan Carlos Rodriguez-Reyes. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.